Abstract
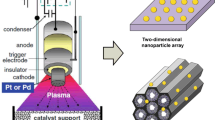
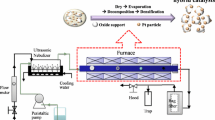
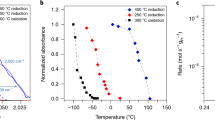
The incorporation of nanosciences into catalysis studies has become the most powerful approach to understanding reaction mechanisms of industrial catalysts and designing new-generation catalysts with high selectivity. Nanoparticle catalysts were synthesized via controlled colloid chemistry routes. Nanostructured catalysts such as nanodots and nanowires were fabricated with nanolithography techniques. Catalytic selectivity is dominated by several complex factors including the interface between active catalyst phase and oxide support, particle size and surface structure, and selective blocking of surface sites, etc. The advantage of incorporating nanosciences into the studies of catalytic selectivity is the capability of separating these complex factors and studying them one by one in different catalyst systems. The role of oxide–metal interfaces in catalytic reactions was investigated by detection of continuous hot electron flow in catalytic nanodiodes fabricated with shadow mask deposition technique. We found that the generation mechanism of hot electrons detected in Pt/TiO2 nanodiode is closely correlated with the turnover rate under CO oxidation. The correlation suggests the possibility of promoting catalytic selectivity by precisely controlling hot electron flow at the oxide–metal interface. Catalytic activity of 1.7–7.2 nm monodispersed Pt nanoparticles exhibits particle size dependence, demonstrating the enhancement of catalytic selectivity via controlling the size of catalyst. Pt–Au alloys with different Au coverage grown on Pt(111) single crystal surface have different catalytic selectivity for four conversion channels of n-hexane, showing that selective blocking of catalytic sites is an approach to tuning catalytic selectivity. In addition, presence and absence of excess hydrogen lead to different catalytic selectivity for isomerization and dehydrocyclization of n-hexane on Pt(111) single crystal surface, suggesting that modification of reactive intermediates by the presence of coadsorbed hydrogen is one approach to shaping catalytic selectivity. Several challenges such as imaging the mobility of adsorbed molecules during catalytic reactions by high pressure STM and removing polymeric capping agents from metal nanoparticles remain.























Similar content being viewed by others
References
Somorjai GA (1994) Introduction to surface chemistry and catalysis. John Wiley & Sons, New York
Yan XM, Kwon S, Contreras AM, Bokor J, Somorjai GA (2005) Nano Lett 5:745
Yan XM, Contreras AM, Koebel MM, Liddle JA, Somorjai GA (2005) Nano Lett 5:1129
Kwon S, Yan XM, Contreras AM, Liddle JA, Somorjai GA, Bokor J (2005) Nano Lett 5:2557
Jacobs PW, Ribeiro FH, Somorjai GA, Wind SJ (1996) Catal Lett 37:131
Jacobs PW, Wind SJ, Ribeiro FH, Somorjai GA (1997) Surf Sci 372:L249
Choi YK, Zhu J, Grunes J, Bokor J, Somorjai GA (2003) J Phys Chem B 107:3340
Zhu J, Somorjai GA (2001) Nano Lett 1:8
Konya Z, Puntes VF, Kiricsi I, Zhu J, Alivisatos AP, Somorjai GA (2002) Nano Lett 2:907
Hoefelmeyer JD, Niesz K, Somorjai GA, Tilley TD (2005) Nano Lett 5:435
Song H, Rioux RM, Hoefelmeyer JD, Komor R, Niesz K, Grass M, Yang PD, Somorjai GA (2006) J Am Chem Soc 128:3027
Rioux RD, Song H, Hoefelmeyer JD, Yang P, Somorjai GA (2005) J Phys Chem B 109:2192
Grunes J (2004) Ph.D. Thesis, University of California, Berkeley
Tsirlin T, Zhu J, Grunes J, Somorjai GA (2002) Top Catal 19:165
Eppler AS, Zhu J, Anderson EA, Somorjai GA (2000) Top Catal 13:33
Choi YK, Zhu J, Grunes J, Bokor J, Somorjai GA (2003) J Phys Chem B 107:3340
Teranishi T, Hosoe M, Tanaka T, Miyake M (1999) J Phys Chem B 103:3818
Wang Y, Ren J, Deng K, Gui L, Tang Y (2000) Chem Mater 12:1622
Ahmadi TS, Wang ZL, Green TC, Henglein A, El-Sayed MA (1996) Science 272:1924
Somorjai GA, Rupprechter G (1999) J Phys Chem 103:1623
Levin ME, Salmeron M, Bell AT, Somorjai GA (1987) J Catal 106:401
Tauster SJ (1987) Acc Chem Res 20:389
Bartholomew CH, Pannell RB, Butler JL (1980) J Catal 65:335
Boffa A, Lin C, Bell AT, Somorjai GA (1994) J Catal 149:149
Somorjai GA (2005) Catal Lett 101:1
Ji X, Zuppero A, Gidwani JM, Somorjai GA (2005) J Am Chem Soc 127:5792
Park JY, Somorjai GA (2006) J Vac Sci Technol B 24:1967
Park JY, Somorjai GA (2006) Chem Phys Chem 7:1409
Schwab GM, Darleth H (1966) J Phys Chem Beue Folge 50:191
Schwab GM, Darleth H (1967) J Phys Chem Neue Folge 53:1
Solymosi F (1967) Catal Rev 1:233
Langenbeck W, Dreyer H, Fuhrman H (1962) J Anorg Chem 314:179
Schwab GM, Putzar R (1962) J Phys Chem (Frankfurt) 31:179
Contreras AM, Grunes J, Yan XM, Liddle A, Somorjai GA (2005) Catal Lett 100:115
Grunes J, Zhu J, Yang M, Somorjai GA (2003) Catal Lett 86:157
Haruta M (1997) Catal Today 36:153
Yang M, Somorjai GA (2004) J Am Chem Soc 126:7698
Yeates RC, Somorjai GA (1987) J Catal 103:208
Sachtler JWA, Somorjai GA (1983) J Catal 81:77
Zaera F, Godbey D, Somorjai GA (1986) J Catal 101:73
Davis SM, Zaera F, Somorjai GA (1984) J Catal 85:206
Yang MC, Chou KC, Somorjai GA (2004) J Phys Chem B 108:14766
Acknowledgement
This work was supported by the Director, Office of Science, Office of Advanced Scientific Computing Research, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Divisions and the Materials Sciences and Engineering Divisions, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Somorjai, G.A., Tao, F. & Park, J.Y. The Nanoscience Revolution: Merging of Colloid Science, Catalysis and Nanoelectronics. Top Catal 47, 1–14 (2008). https://doi.org/10.1007/s11244-007-9028-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-007-9028-1