Abstract
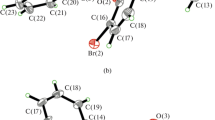
Four oxovanadium(IV) complexes, namely [VO(desa-met)(phen)]·MeOH·2H2O (1) (desa-met = Schiff base derived from 4-(diethylamino)salicylaldehyde and dl-methionine, phen = 1,10-phenanthroline), [VO(o-van-met) (phen)]·MeOH·CH2Cl2·3H2O (2) (o-van-met = Schiff base derived from o-vanillin and dl-methionine), [VO(dtbs-napa)(phen)]·2H2O (3) (dtbs-napa = Schiff base derived from 3,5-di-tert-butyl salicylaldehyde and 3-(1-naphthyl)-l-alanine) and [VO(hyna-napa)(phen)]·1.5H2O (4) (hyna-napa = Schiff base derived from 2-hydroxy-1-naphthaldehyde and 3-(1-naphthyl)-l-alanine), were synthesized and characterized by IR, HRMS, UV–vis spectra, molar conductance and single-crystal X-ray diffraction (XRD). X-ray structural analysis showed that the V(IV) atoms in all four complexes are six-coordinated in a distorted octahedral environment. In the crystals of complexes 1 and 2, π–π stacking interactions together with hydrogen bonds connect the molecular units into 2D networks. Meanwhile, CH–π stacking interactions are observed between the aromatic rings in the crystals of 1 and 4, while the π–π stacking interactions between aromatic rings in the crystals of 2 and 3 are arranged with a face-to-face mode. The in vitro anticancer activities of these complexes against A-549 and HeGp2 cells were tested by MTT assay.











Similar content being viewed by others
References
Wilson JJ, Lippard SJ (2012) J Med Chem 55:5326–5336
Jany T, Moreth A, Gruschka C, Sischka A, Spiering A, Dieding M, Wang Y, Samo SH, Stammler A, Bogge H, Mollard GF, Anselmetti D, Glaser T (2015) Inorg Chem 54:2679–2690
Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G (2012) Oncogene 31:1869–1883
Pabla N, Dong Z (2008) Kidney Int 73:994–1007
Miller RP, Tadagavadi RK, Ramesh G, Reeves WB (2010) Toxins 2:2490–2518
Aher SB, Muskawar PN, Thenmozhi K, Bhagat PR (2014) Eur J Med Chem 81:408–419
Wanninger S, Lorenz V, Subhanb A, Edelmann FT (2015) Chem Soc Rev 44:4986–5002
Almodares Z, Lucas SJ, Crossley BD, Basri AM, Pask CM, Hebden AJ, Phillips RM, McGowan PC (2014) Inorg Chem 53:727–736
Yuan ZL, Shen XM, Huang JD (2015) RSC Adv 5:10521–10528
Yuan ZL, Shen XM, Huang JD, Gang W (2015) J Incl Phenom Macrocycl Chem 82:135–143
Trachootham D, Alexandre J, Huang P (2009) Nat Rev Drug Discov 8:579–591
Barrio DA, Etcheverry SB (2010) Curr Med Chem 17:3632–3642
Strianese M, Basile A, Mazzone A, Morello S, Turco MC, Pellecchia C (2013) J Cell Physiol 228:2202–2209
Evangelou AM (2002) Crit Rev Oncol Hematol 42:249–265
Ebrahimipour SY, Sheikhshoaie I, Kautz AC, Ameri M (2015) Polyhedron 93:99–105
Yang J, Yuan ZL, Yu GQ, He SL, Hu QH, Wu Q, Jiang B, Wei G (2015) J. Fluorescence 26:43–51
Xu YJ, Keiichi K, Motomu K, Masakatsu S, Shigeki M (2014) J Am Chem Soc 136:9190–9194
Chow MJ, Licona C, Wong DYQ, Pastorin G, Gaiddon C, Ang WH (2014) J Med Chem 57:6043–6059
Cao YP, Yi QL, Liu HM, Li HX, Zuo JL, Yuan ZL (2015) Chin J Synth Chem 23:1124–1129
Sapana K, Ghanshyam SC (2014) ACS Appl Mater Interfaces 6:5908–5917
Yuan ZL, Yang QWuXB, Hu QH, Zhang MQ (2011) Chin J Org Chem 31:1698–1702
Hina Z, Anis A, Asad UK, Tahir AK (2015) J Mol Struct 1097:129–135
Zeinab MS, Amini Z, Davar MB, Notash B (2013) Polyhedron 53:76–82
Ozaki Y, Kawashima T, Abe-Yoshizumi R, Kandori H (2014) Biochemistry 53:6032–6040
CrystalClear (2000) Version 1.36, Molecular Structure Corp and Rigaku Corp., The Woodlands
Sheldrick GM SHELXS 97, program for crystal structure solution. University of Göttingen, Göttingen
Sasmal PK, Patra AK, Nethaji M, Chakravarty AR (2007) Inorg Chem 46:11112–111121
Sheng GH, Han X, You ZL, Li HH, Zhu HL (2014) J Coord Chem 67:1760–1770
Vančo J, Trávníek Marek J Z, Račanská E, Muselík J, Vajlenová O (2008) J Inorg Biochem 102:595–605
Neelakantan MA, Rusalraj F, Dharmaraja J, Johnsonraja S, Jeyakumar T, Pillai MS (2008) Spectrochim Acta A 71:1599–1609
Bian L, Li LZ, Zhang QF, Dong JF, Xu T, Li JH, Kong JM (2012) Trans Met Chem 37:783–790
Habala L, Bartel C, Giester G, Jakupec MA, Keppler BK, Rompel A (2015) J Inorg Biochem 147:147–152
Cao YP, Yi QL, Liu HM, Li HX, Zuo JL, Yuan ZL (2015) J Zunyi Med Univ 38(6):584–590
Balaji B, Somyajit K, Banik B, Nagaraju G, Chakravarty AR (2013) Inorg Chim Acta 400:142–150
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81360471), the International Cooperation Project of Guizhou Province (No. [2012]7036), Science and the ‘Chunhui’ plan project of Ministry of Education (No. Z2014089) and the National Project of Training Programs of Innovation and Entrepreneurship for Undergraduates, China (No. 201510661007).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, Y., Yi, C., Liu, H. et al. Syntheses, crystal structures and in vitro anticancer activities of oxovanadium(IV) complexes of amino acid Schiff base and 1,10-phenanthroline ligands. Transit Met Chem 41, 531–538 (2016). https://doi.org/10.1007/s11243-016-0049-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-016-0049-0