Abstract
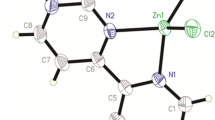
Under different solvothermal conditions, the reactions of 2-ppds (2-ppds = di[4-(pyridin-2-yl)pyrimidinyl]disulfide) with CuX2 (X = ClO4 or Cl) produced markedly different results because of diverse in situ reactions of 2-ppds involving dynamic S–S and C–S bond cleavage. At 90 °C, reaction of 2-ppds with Cu(ClO4)2 in CH3CN–CH2Cl2-mixed solvent yielded a discrete mononuclear complex, [Cu(L1)2(ClO4)]ClO4 (1), in which 2-ppds was converted into a zwitterion of L1 (L1 = 4-(pyridin-2-yl)pyrimidin-2-ol) involving C–S bond scission followed by attack of water. At 120 °C in DMF–MeOH solvent, reaction of 2-ppds with Cu(ClO4)2 resulted in transformation to L2 (L2 = 4-(pyridin-2-yl)pyrimidine-2-thiolate) through reductive cleavage of the S–S bond concurrent with reduction of Cu2+ to Cu+, leading to the formation of a single tetranuclear coordination complex, [Cu4(L2)4] (2), that comprises a unique Cu4S4 cluster. When the reaction between 2-ppds and CuCl2 was carried out at 90 °C in CH3CN–H2O solvent, a discrete coordination complex, [Cu(L3)(H2O)Cl]Cl (3), was obtained, resulting from conversion of 2-ppds into L3 (L3 = bis(4-(pyridin-2-yl)pyrimidin-2-yl)sulfane) through extrusion of one S atom from the S–S bond.




Similar content being viewed by others
References
Zhang XM (2005) Coord Chem Rev 249:1201
Chen XM, Tong ML (2007) Acc Chem Res 40:162
Zhu HB, Gou SH (2011) Coord Chem Rev 255:318
Aragoni MC, Arca M, Crespo M, Devillanova FA, Hursthouse MB, Huth SL, Isaia F, Lippolis V, Verani G (2007) CrystEngCommun 9:874
Carballo R, Covelo B, Fernández-Hermida N, Lago AB, Vázquez-López EM (2009) CrystEngCommun 11:817
Delgado S, Molina-Ontaoria A, Medina ME, Pastor CJ, Jiménez-Aparicio R, Priego JL (2006) Inorg Chem Commun 9:1289
Delgado S, Barrilero A, Molina-Ontoria A, Medina ME, Pastor CJ, Jiménez-Aparicio R, Priego JL (2006) Eur J Inorg Chem 2746
Yoo HS, Yoon JH, Kim JI, Koh EK, Hong CS (2008) Eur J Inorg Chem 3123
la Pinta ND, Caballero AB, Madariaga G, Ezpeleta JM, Rodriguez-Dieguez A, Salas JM, Cortés R (2014) CrystEngComm 16:8322
Lumb I, Hundal MS, Hundal G (2014) Inorg Chem 54:7770
Han L, Bu X, Zhang Q, Feng P (2006) Inorg Chem 45:5736
Wang J, Zhang YH, Li HX, Lin ZJ, Tong ML (2007) Cryst Growth Des 7:2352
Chen Y, Wang ZQ, Ren ZG, Li HX, Li DX, Liu D, Zhang Y, Lang JP (2009) Cryst Growth Des 9:4963
Wang J, Zheng SL, Hu S, Zhang YH, Tong ML (2007) Inorg Chem 46:795
Ma LF, Wang YY, Wang LY, Lu DH, Batten SR, Wang JG (2009) Cryst Growth Des 9:2036
Delgado S, Sanz Miguel PJ, Priego JL, Jiménez-Aparicio R, Gómez-García CJ, Zamora F (2008) Inorg Chem 47:9128
Zhang YN, Wang YY, Hou L, Liu P, Liu JQ, Shi QZ (2010) CrystEngComm 12:3840
Zhu QL, Sheng TL, Tan CH, Hu SM, Fu RB, Wu XT (2011) Inorg Chem 50:7618
Gallego AL, Castillo O, Zamora F, Delgado S (2013) RSC Adv 3:18406
Gallego A, Castillo O, Gómez-García CJ, Zamora F, Delgado S (2012) Inorg Chem 51:718
Delgado S, Gallego A, Castillo O, Zamora F (2011) Dalton Trans 40:847
Zhu HB, Li L, Xu G, Gou SH (2010) Eur J Inorg Chem 1143
Zhu HB, Lu X, Yang WN, Gou SH (2012) Polyhedron 31:801
Zhu HB, Wu YF, Zhao Y, Hu J (2014) Dalton Trans 43:17156
SAINT, version 6.02a, Bruker AXS Inc., Madison, WI, 2002
Sheldrick GM (1997) SADABS, Program for Bruker area detector absorption correction, University of Göttingen, Göttingen, Germany, 1997
Sheldrick GM (1997) SHELXL-97, Program for crystal structure refinement; University of Göttingen, Göttingen, Germany, 1997
Huang CH, Gou SH, Zhu HB, Huang W (2007) Inorg Chem 46:5537
Huang XC, Zhang JP, Lin YY, Yu XL, Chen XM (2004) Chem Comm 1100
Zhu HB, Li L, Wang H, Lu X, Gou SH (2010) Inorg Chem Comm 13:30
Acknowledgments
We are grateful for the financial support from National Natural Science Foundation of China (NSFC) (Nos. 21171036 and 20801011) and the Fundamental Research Funds for the Central Universities (No. 3207045415).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, HB., Yao, G. & Li, WS. Solvothermal heterocyclic disulfide/CuX2 (X = Cl, ClO4) reactions involving dynamic S–S and C–S bond cleavage. Transition Met Chem 41, 57–63 (2016). https://doi.org/10.1007/s11243-015-9996-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9996-0