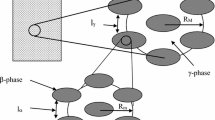
Abstract
Ion storage in porous electrodes is important in applications such as energy storage by supercapacitors, water purification by capacitive deionization, extraction of energy from a salinity difference and heavy ion purification. A model is presented to simulate the charge process in homogeneous porous media comprising big pores. It is based on a theory for capacitive charging by ideally polarizable porous electrodes without faradaic reactions or specific adsorption of ions. A volume averaging technique is used to derive the averaged transport equations in the limit of thin electrical double layers. Transport between the electrolyte solution and the charged wall is described using the Gouy–Chapman–Stern model. The effective transport parameters for isotropic porous media are calculated solving the corresponding closure problems. The source terms that appear in the average equations are calculated using numerical computations. An alternative way to deal with the source terms is proposed.







Similar content being viewed by others
Abbreviations
- \(A_{\upalpha \upbeta }\) :
-
Interphase area \(\upalpha \)–\(\upbeta \) (m\(^{2}\))
- \(a_{v}\) :
-
Effective area (m\(^{2}\,\)m\(^{-3})\)
- \(c_{i}\) :
-
Ion concentration (mol m\(^{-3})\)
- \(C_{\infty }\) :
-
Salt bulk concentration (mol m\(^{-3})\)
- \({\tilde{c}}_\upalpha \) :
-
Deviation salt concentration in \(\upalpha \)-phase (mol m\(^{-3})\)
- \(\langle c_\upalpha \rangle ^{\upalpha }\) :
-
Intrinsic phase average concentration (mol m\(^{-3})\)
- \({\underline{\underline{D}}}_\mathrm{eff}\) :
-
Effective diffusivity tensor (m\(^{2}\,\mathrm{s}^{-1})\)
- \(D_{i}\) :
-
Diffusion coefficient (m\(^{2}\,\hbox {s}^{-1})\)
- \(\underline{f}_{1}\) :
-
Closure vector field (m)
- \(f_{2}\) :
-
Closure scalar field (s m\(^{-1})\)
- \(F\) :
-
Faraday constant (C mol\(^{-1})\)
- \(\underline{g}_{1}\) :
-
Closure vector field (m)
- \(g_{2}\) :
-
Closure scalar field \((\hbox {V m}^{2}\,\hbox {s mol}^{-1})\)
- \({\underline{\underline{I}}}\) :
-
Unit tensor (dim.)
- \(\underline{I}_{e}\) :
-
Ionic current per unit area \((\hbox {A m}^{-2})\)
- \(J_\mathrm{charge}\) :
-
Charge-transfer flux \((\hbox {C m}^{-2}\,\hbox {s}^{-1})\)
- \(J_\mathrm{salt}\) :
-
Salt molar flux \((\hbox {mol m}^{-2}\,\hbox {s}^{-1})\)
- \(l_{\upalpha }\) :
-
Microscopic length scale (m)
- \(\underline{l}_{i}\) :
-
Lattice vectors (m)
- \(L\) :
-
Macroscopic length scale (m)
- \(L_{c}\) :
-
Macroscopic length scale for the gradient (m)
- \(\underline{n}_{\upalpha \upbeta }\) :
-
Unit normal vector from the \(\upalpha \) into the \(\upbeta \)-phase (dim.)
- \(N_{i}\) :
-
Ion flux \((\hbox {mol m}^{-2}\,\hbox {s}^{-1})\)
- \(q\) :
-
Excess charge density \((\hbox {C m}^{-2})\)
- \(\langle q\rangle _{\upalpha \upbeta }\) :
-
Excess charge area averaged \((\hbox {C m}^{-2})\)
- \(r_{o}\) :
-
Radius of the representative elementary volume (m)
- \(t\) :
-
Time (s)
- \(t_{C}^{\bullet }\) :
-
Characteristic time for the supercapacitor regime (s)
- \(t_{D}^{\bullet }\) :
-
Characteristic time for the desalination regime (s)
- \(u_{i}\) :
-
Isotropic mobility \((\hbox {m}^{2}\,\hbox {V}^{-1}\,\hbox {s}^{-1})\)
- \({\underline{\underline{U}}}_\mathrm{eff}\) :
-
Mobility tensor \((\hbox {m}^{2}\,\hbox {V}^{-1}\,\hbox {s}^{-1})\)
- \(V_{\upalpha }\) :
-
\(\upalpha \)-Phase in REV \((\hbox {m}^{3})\)
- \(V_\mathrm{T}\) :
-
Thermal voltage (V)
- \(w\) :
-
Excess salt density \((\hbox {mol m}^{-2})\)
- \(\langle w\rangle _{\upalpha \upbeta }\) :
-
Excess salt adsorption area average \((\hbox {mol m}^{-2})\)
- \(x\) :
-
Spatial position (m)
- \(z_{i}\) :
-
Ionic charge number (dim.)
- \(\Delta \phi _\mathrm{D}\) :
-
Diffuse-layer potential difference (V)
- \(\Delta \phi _\mathrm{Stern}\) :
-
Stern-layer potential difference (V)
- \(\varepsilon _{\upalpha }\) :
-
\(\upalpha \)-Phase volume fraction (dim.)
- \(\phi \) :
-
Electrostatic potential (V)
- \(\tilde{\phi }_\upalpha \) :
-
Potential deviation in \(\upalpha \)-phase (V)
- \(\langle \phi _\upalpha \rangle ^{\upalpha }\) :
-
Intrinsic phase average potential (V)
- \(\kappa \) :
-
Effective conductivity \((\hbox {A V}^{-1}\,\hbox {m}^{-1})\)
- \(\lambda _\mathrm{B}\) :
-
Bjerrum length (m)
- \(\lambda _\mathrm{D}\) :
-
Debye length (m)
- \(\psi _{\upalpha }\) :
-
Generic variable in \(\upalpha \)-phase
- \(\underline{\nabla }\) :
-
Nabla operator \((\hbox {m}^{-1})\)
- \(i\) :
-
Component \(i\)
- \(\upalpha \) :
-
\(\upalpha \)-Phase
- \(\upbeta \) :
-
\(\upbeta \)-Phase
- \(\upalpha \upbeta \) :
-
\(\upalpha \)–\(\upbeta \) interphase
- \(\upalpha \) :
-
\(\upalpha \)-Phase
- \(\upbeta \) :
-
\(\upbeta \)-Phase
References
Anderson, M.A., Cudero, A.L., Jesus Palma, J.: Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: will it compete? Electrochim. Acta 55, 3845–3856 (2010)
Bazant, M.Z., Thornton, K., Ajdari, A.: Diffuse–charge dynamics in electrochemical systems. Phys. Rev. E 70, 021506 (2004)
Biesheuvel, P.M., Bazant, M.Z.: Nonlinear dynamics of capacitive charging and desalination by porous electrodes. Phys. Rev. E 81, 031502 (2010)
Biesheuvel, P.M., van Soestbergen, M., Bazant, M.Z.: Imposed currents in galvanic cells. Electrochim. Acta 54, 4857 (2009)
Biesheuvel, P.M., Yu, F., Bazant, M.Z.: Diffuse charge and Faradaic reactions in porous electrodes. Phys. Rev. E 83, 061507 (2011)
Biesheuvel, P.M., Fu, Y., Bazant, M.Z.: Electrochemistry and capacitive charging of porous electrodes in asymmetric multicomponent electrolytes. Russ. J. Electrochem. 48, 580 (2012)
Biesheuvel, P.M., Porada, S., Levi, M., Bazant, M.Z.: Attractive forces in microporous carbon electrodes for capacitive deionization. J. Solid State Electrochem. 18, 1365 (2014)
Bockris, J.O., Reddy, A.K.N., Gamboa-Aldeco, M.: Modern Electrochemistry: Fundamentals of Electrodics, vol. 2b. Plenum Press, New York (2000)
Borges da Silva, E.A., Souza, D.P., Ulson de Souza, A.A., Guelli U. de Souza, S.M.A.: Prediction of effective diffusivity tensors for bulk diffusion with chemical reactions. Braz. J. Chem. Eng. 24, 47–60 (2007)
Brenner, H.: Dispersion resulting from flow through spatially periodic porous media. Trans. R. Soc. 297, 81–133 (1980)
Buyuktas, D., Wallender, W.W.: Dispersion in spatially periodic porous media. Heat Mass Transf. 40, 261–270 (2004)
Carbonell, R.G., Whitaker, S.: Dispersion in pulsed systems—II Theoretical developments for passive dispersion in porous media. Chem. Eng. Sci. 38, 1795–1802 (1983)
Carbonell, R.G., Whitaker, S.: Heat and Mass Transfer in Porous Media. In: Bear, J., Corapcioplu, M.Y. (eds.) Fundamentals of Transport Phenomena in Porous Media, p. 121. Marinus Nijhoff, Dordrecht (1984)
Chu, K.T., Bazant, M.Z.: Nonlinear electrochemical relaxation around conductors. Phys. Rev. E 74, 011501 (2006)
Crapiste, G.H., Rotstein, E., Whitaker, S.: A general closure scheme for the method of volume averaging. Chem. Eng. Sci. 41, 227–235 (1986)
Currie, J.A.: Gaseous diffusion in porous media. Part 2—Dry granular materials. Br. J. Appl. Phys. 11, 318 (1960)
Gabitto, J.F.: Effect of the microstructure on anisotropic diffusion in porous media. Int. Commun. Heat Mass Transf. 18, 459–466 (1991)
Gray, W.G.: A derivation of the equations for multiphase transport. Chem. Eng. Sci. 30, 229 (1975)
Gray, W.G., Lee, P.C.Y.: On the theorems for local volume averaging of multiphase systems. Int. J. Multiphase Flow 3, 333–340 (1977)
Johnson, A.M., Newman, J.: Desalting by means of carbon electrodes. J. Electrochem. Soc. 118, 510–517 (1971)
Kim, J.H., Ochoa, J.A., Whitaker, S.: Diffusion in anisotropic porous media. Transp. Porous Media 2, 327–356 (1987)
Levich, V.G.: Physicochemical Hydrodynamics. Prentice-Hall, Englewood Cliffs (1962)
Liu, H.-J., Wang, X.-M., Cui, W.-J., Dou, Y.-Q., Zhao, D.-Y., Xia, Y.Y.: Highly ordered mesoporous carbon nanofiber arrays from a crab shell biological template and its application in supercapacitors and fuel cells. J. Mater. Chem. 2010(20), 4223–4230 (2010)
Locke, B.R.: Electrophoretic transport in porous media: a volume-averaging approach. Ind. Eng. Chem. Res. 37, 615–625 (1998)
Mathioulakis, E., Belessiotis, V., Delyannis, E.: Desalination by using alternative energy: review and state of the art. Desalination 203, 346–365 (2007)
Newman, J., Thomas-Alyea, K.E.: Electrochemical Systems, 3rd edn. Wiley, New York (2004). (Chap. 22)
Nozad, I., Carbonell, R.G., Whitaker, S.: Heat conduction in multiphase systems—I. Theory and experiment for two-phase systems. Chem. Eng. Sci. 40, 843 (1985)
Ochoa-Tapia, J.A., Stroeve, P., Whitaker, S.: Diffusive transport in two-phase media: Spatially periodic models and Maxwell’s theory for isotropic and anisotropic systems. Chem. Eng. Sci. 49, 709–726 (1994)
Ochoa-Tapia, J.A., Del Rio, J.A., Whitaker, S.: Bulk and surface diffusion in porous media: an application of the surface-averaging theorem. Chem. Eng. Sci. 48, 2061–2082 (1993)
Paine, M.A., Carbonell, R.G., Whitaker, S.: Dispersion in pulsed systems—I Heterogeneous reaction and reversible adsorption in capillary tubes. Chem. Eng. Sci. 38, 1781–1793 (1983)
Pivonka, P., Smith, D., Gardiner, B.: Investigation of Donnan equilibrium in charged porous materials—a scale transition analysis. Transp. Porous Media 69, 215–237 (2007)
Pivonka, P., Narsilio, G., Li, R., Smith, D., Gardiner, B.: Electrodiffusive transport in charged porous media: from the particle-level scale to the macroscopic scale using volume averaging. J. Porous Media 12, 101–118 (2009)
Porada, S., Zhao, R., Van der Wal, A., Presser, V., Biesheuvel, P.M.: Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 58, 1388–1442 (2013)
Quintard, M.: Diffusion in isotropic and anisotropic porous systems: three-dimensional calculations. Transp. Porous Media 11, 187–199 (1993)
Quintard, M., Whitaker, S.: Transport in chemical and mechanical heterogeneous porous media IV: large-scale mass equilibrium for solute transport with adsorption. Adv. Water Resour. 22, 33–57 (1998)
Ryan, D., Carbonell, R.G., Whitaker, S.: Effective diffusivities for catalyst pellets under reactive conditions. Chem. Eng. Sci. 35, 10–16 (1980)
Satterfield, C.N.: Mass Transfer in Heterogeneous Catalysis. MIT Press, Cambridge (1970)
Sharma, K., Mayes, R.T., Kiggans Jr, J.O., Yiacoumi, S., Gabitto, J., DePaoli, D.W., Dai, S., Tsouris, C.: Influence of temperature on the electrosorption of ions from aqueous solutions using mesoporous carbon materials. Sep. Purif. Technol. 116, 206–213 (2013)
Scheiner, S., Pivonka, P., Smith, D.: Two-scale model for electro-diffusive transport through charged porous materials. In: IOP Conference Series: Materials Science and Engineering, vol. 10, p. 012112 (2010)
Shiklomanov, I.: World fresh water resources. In: Gleick, P.H. (ed.) Water in Crisis: A Guide to the World’s Fresh Water Resources. Oxford University Press, New York (1993)
Spiegler, K.S., El-Sayed, Y.M.: The energetics of desalination processes. Desalination 134, 109–128 (2001)
Tiedemann, W., Newman, J.: Porous-electrode theory with battery applications. AIChE J. 21, 25 (1975)
Tsouris, C., Mayes, R., Kiggans, J., Sharma, K., Yiacoumi, S., DePaoli, D., Dai, S.: Mesoporous carbon for capacitive deionization of saline water. Environ. Sci. Technol. 45, 10243–10249 (2011)
USGS: Distribution of Earth’s water, taken from USGS website: http://water.usgs.gov/edu/earthwherewater.html, 17 March 2014
Ulson de Souza, A.A., Whitaker, S.: The modeling of a textile dyeing process utilizing the method of volume averaging. Braz. J. Chem. Eng. 20, 445–453 (2003)
Valdes-Parada, F.J., Alvarez-Ramirez, J.: On the effective diffusivity under chemical reaction in porous media. Chem. Eng. Sci. 65, 4100–4104 (2010)
Villar, I., Roldan, S., Ruiz, V., Granda, M., Blanco, C., Menendez, R., Ricardo Santamarıa, R.: Capacitive deionization of NaCl solutions with modified activated carbon electrodes. Energy Fuels 24, 3329–3333 (2010)
Whitaker, S.: Local thermal equilibrium: an application to packed bed catalytic reactor design. Chem. Eng. Sci. 41, 2029 (1986)
Whitaker, S.: The Method of Volume Averaging. Kluwer, Dordrecht (1999)
Zhao, R., Biesheuvel, P.M., Miedema, H., Brunning, H., van de Wal, A.: Charge efficiency: a functional tool to probe the double-layer structure inside of porous electrodes and application in the modeling of capacitive deionization. J. Phys. Chem. Lett. 1, 205–210 (2010)
Zhou, L., Li, L., Song, H., Morris, G.: Using mesoporous carbon electrodes for brackish water desalination. Water Res. 42, 2340–2348 (2008)
Acknowledgments
This research was partially conducted at the Oak Ridge National Laboratory (ORNL) and supported by the Laboratory Director’s Research and Development Seed Program of ORNL. ORNL is managed by UT-Battelle, LLC, under Contract DE-AC05-0096OR22725 with the US Department of Energy.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
1.1 Order of Magnitude Analysis in Eq. (18)
Using, \(- \underline{n}_{\upalpha \upbeta }\bullet \underline{\nabla }{f}_2 = 1/D\), we get \({f}_2 \approx O({l}_\upalpha /D)\) because \(f_{2}\) varies with \(l_{\upalpha }\). From \(\underline{{u}}=\frac{{1}}{{V}_{\upalpha }}\int \limits _{{A}_{{\upalpha \upbeta }}} {{\underline{{n}}}_{{\upalpha \upbeta }} {f}_{2} { \hbox { d}A}} \), using mean value theorem and \({f}_2 \approx O({l}_\upalpha /D)\) we get, \(\underline{{u}}\approx O{(1 / D)}\). Here, we used \(A/V_{\upalpha } = a_{V}\) and \({a}_{V} \approx O({l}_\upalpha ^{{-1}})\).
1.2 Order of Magnitude Analysis in Eq. (32)
The order of magnitude for the deviation variables \({\tilde{c}}_{\upalpha } \) and \(\tilde{\phi }_{\upalpha } \) were obtained using Eqs. (23) and (36). We use \({g}_2 \approx O\left( {l}_\upalpha / \left( U{\left\langle {{c}_{\upalpha }} \right\rangle }^\upalpha \right) \right) \) estimated from Eq. (41).
Rights and permissions
About this article
Cite this article
Gabitto, J., Tsouris, C. Volume Averaging Study of the Capacitive Deionization Process in Homogeneous Porous Media. Transp Porous Med 109, 61–80 (2015). https://doi.org/10.1007/s11242-015-0502-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-015-0502-0