Abstract
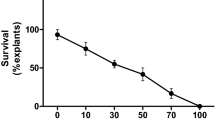
Shoot tip explants prepared from seedlings of ML-267 genotype of green gram were inoculated on MSB5 medium supplemented with BAP (0–20 μM) individually or in combination with minimal concentration of auxins (NAA/IAA/IBA) for adventitious shoots formation. BAP alone without auxins was observed to be efficient in multiple shoot induction and optimum shoot proliferation was achieved on MSB5 medium containing 10 μM BAP with 100 % shoot induction frequency. 3-day-old explants gave best shoot multiplication response and the mean shoot number decreased significantly in 4-day and 5-day-old explants. The induced shoots rooted profusely on ½ MSB5 + 2.46 µM IBA and about 90 % of the plantlets survived after acclimatization and set seed normally. Shoot tip explants infected with A.tumefaciens (LBA4404) harboring pCAMBIA 2301 + AnnBj1 recombinant vector. Various factors which influence the competence of transformation were optimized based on the frequency of transient GUS expression in shoot tip explants. Optimum levels of transient GUS expression were recorded at pre-culture of explants for 2 days, infection for 10 min with Agro-culture of 0.8 OD and co-cultivation for 3 days on co-cultivation medium containing 100 µM acetosyringone in dark at 23 °C. Putative transformed shoots were produced on selection medium (shoot inductionmedium with100 mg/l kanamycin and 250 mg/l cefotaxim). PCR analysis confirmed the presence of AnnBj1, nptII, and uidA genes in T0 plants. Stable GUS activity was detected in flowers of T0 plants and leaves of T1 plants. PCR analysis of T1 progeny revealed AnnBj1 gene segregated following a Mendelian segregation pattern.










Similar content being viewed by others
Abbreviations
- BAP:
-
6-Benzyleaminopurine
- Cefo:
-
Cefotaxim
- GUS:
-
β-Glucuronidase
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-buteric acid
- Kan:
-
Kanamycin
- MSB5:
-
MS basal salts with gamborg vitamines
- NAA:
-
1-Naphthaleneacetic acid
- OD:
-
Optical density
- SBIM:
-
Shoot bud induction medium
References
Aldemita RR, Hodges TK (1996) Agrobacterium tumefaciens mediated-transformation of japonica and indica rice varieties. Planta 199:612–617
Amutha S, Muruganantham M, Ganapathi A (2006) Thidiazuron induced high frequency axillary and adventitious shoot regeneration in Vigna radiata (L.) Wilczek. In Vitro Cell Dev Biol-Plant 42:26–30
Anuradha TS, Jami SK, Datla RS, Kirti PB (2006) Genetic transformation of peanut (Arachis hypogaea L.) using cotyledonary node as explant and a promoter less gus::nptII fusion gene based vector. J Biosci 31:235–246
Aragao FJL, Ribeiro SG, Barros LMG, Brasileiro ACM, Maxwell DP, Rech EL, Faria JC (1998) Transgenic beans (Phaseolus vulgaris L.) engineered to express viral antisense RNAs show delayed and attenuated symptoms of bean golden mosaic geminivirus. Mol Breed 4:491–499
Avenido RA, Hautea DM (1990) In vitro organogenesis and flowering in mungbean (V. radiata L. Wilczek). Philipp J Crop Sci 15:169–173
Avenido RA, Motoda J, Hattori K (2001) Direct shoot regeneration from cotyledonary nodes as a marker for genomic groupings within the Asiatic Vigna (subgenus Ceratotropis (Piper) Verdc) species. Plant Growth Regulat 35:59–67
Baron C, Zambryski PC (1995) The plant response in pathogenesis, symbiosis and wounding: variations on a common theme. Ann Rev Genet 29:107–129
Beena MR, Jami SK, Srinivasan T, Swathi Anuradha T, Padmaja G, Kirti PB (2005) Efficient regeneration from cotyledonary node explants of peanut (Arachis hypogeal L. cv. JL-24). Indian J Plant Physi 15:131–134
Brar MS, Anderson EJ (1997) In vitro shoot tip multiplication of cowpea. In Vitro Cell Dev Biol-Plant 33:114–118
Chakraborty J, Sen S, Ghosh P, Sengupta A, Basu D, Das S (2016) Homologus promoter derived constitutive and chloroplast targeted expression of synthetic cry1Ac in transgenic chickpea confers resistance against Helicoverpa armigera. Plant Cell Tiss Org Cult 125:521–535
Chandra A, Pental D (2003) Regeneration and genetic transformation of grain legumes: an overview. Curr Sci 84:381–387
Dita MA, Rispail N, Prats E, Rubiales D, Singh KB (2006) Biotechnology approaches to overcome biotic and abiotic stress constraints in legumes. Euphytica 147:1–24
Eapen S (2008) Advances in development of transgenic pulse crops. Biotech Adv 26:162–168
Franklin CI, Trieu TN, Gonzales RA, Dixon RA (1991) Plant regeneration from seedling explants of green bean (Phaseolus vulgaris L.) via organogenesis. Plant Cell Tiss Org Cult 24:199–206
Geetha N, Venkatachalam P, Sita GL (1999) Agrobacterium-mediated genetic transformation of pigeonpea (Cajanus cajan L.) and development of transgenic plants via direct organogenesis. Plant Biotechnol 16:213–218
Geng L, Niu L, Gresshoff PM, Shu C, Sing F, Huang D, Zhang J (2012) Efficient production of Agrobacterium rhizogenes-transformed roots and composite plants in peanut (Arachis hypogaea L.). Plant Cell Tiss Org Cult 109:491–500
Girija S, Ganapathi A, Ananthakrishnan G (2000) Somatic embryogenesis in Vigna radiata (L.) Wilczek. Indian J Exp Biol 38:1241–1244
Gulati A, Jaiwal PK (1992) In vitro induction of multiple shoots and plant regeneration from shoot tips of mungbean (Vigna radiata (L.) Wilczek). Plant Cell Tiss Org Cult 29:199–205
Gulati A, Jaiwal PK (1994) Plant regeneration from cotyledonary node explants of mungbean (Vigna radiata (L.) Wilczek). Plant Cell Rep 13:523–527
Halder M, Jha S (2016) Enhanced trans-resveratrol production in genetically transformed root cultures of peanut (Arachis hypogaea L.) Plant Cell Tiss Org Cult 124:555–572
Himabindu Y, Reddy MC, Chandrasekhar T (2014) In vitro regeneration of green gram (Vigna radiata (L.) Wilczek) cultivar Vamban-2 using cotyledonary nodes. CIBTech J Biotech 3:11–15
Jackson JA, Hobbs SLA (1990) Rapid multiple shoot production from cotyledonary node explants of pea (Pisum sativum L.) In Vitro Cell Dev Biol-Plant 26:835–838
Jaiwal PK, Gulati A (1995) Current status and future strategies of in vitro culture techniques for genetic improvement of mung bean (Vigna radiata (L.) Wilczek). Euphytica 86:167–181
Jaiwal PK, Kumari R, Ignacimuthu S, Potrykus I, Sautter C (2001) Agrobacterium tumefaciens-mediated gene transfer in mungbean-a recalcitrant grain legume. Plant Sci 161:239–247
Jefferson RA, Kavanagh TA, Bevan MW (1987) Gus fusion: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jin T, Chang Q, Li W, Yin D, Li Z, Wang D, Liu B, Liu L (2010) Stress-inducible expression of GmDREB1 conferred salt tolerance in transgenic alfalfa. Plant Cell Tiss Org Cult 100:219–227
Kaviraj CP, Kiran G, Venugopal RB, Kishore PBK, Rao S (2006) Somatic embryogenesis and plant regeneration from cotyledonary explants of green gram (Vigna radiata (L.) Wilczek)-a recalcitrant grain legume. In Vitro Cell Dev Biol-Plant 42:134–138
Kim MJ, Baek K, Park CM (2009) Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep 28:1159–1167
Krishna G, Reddy PS, Ramteke PW, Rambabu P, Tawar KB, Bhattacharya P (2011) Agrobacterium-mediated genetic transformation of pigeon pea (Cajanus cajan (L.) Millsp.) for resistance to legume pod borer Helicoverpa armigera. J Crop Sci Biotech 14:197–204
Lawrence PK and Koundal KR (2001) Agrobacterium tumifaciens-mediated transformation of pigeon pea (Cajanus cajan (L) Millsp) and molecular analysis of regenerated plants. Curr Sci 80:1428–1432
Li HY, Zhu YM, Chen Q, Conner RL, Ding XD, Li J, Zhang BB (2004) Production of soybean transgenic plants with two anti-fungal protein genes via Agrobacterium and particle bombardment. Biol Plant 48:367–374
Liu M, LiD, Wang Z, Meng F, Li Y, Wu X, Teng W, Han Y, Li W (2012) Transgenic expression of ThIPK2 gene in soybean improves stress tolerance, oleic acid content and seed size. Plant Cell Tiss Org Cult 111:277–289
Mahalakshmi LS, Leela T, Kumar SM, Kumar BK, Naresh B, Devi P (2006) Enhanced genetic transformation efficiency of mungbean by use of primary leaf explants. Curr Sci 91:93–99
Mohan KL, Krishnamurthy KV (2003) Plant regeneration from decapitated mature embryo axis and Agrobacterium-mediated genetic transformation of pigeon pea. Biol Plant 46:519–527
Muthukumar B, Mariamma M, Veluthambi K, Gnanam A (1996) Genetic transformation of cotyledon explants of cowpea (Vigna unguiculata (L.) Walp) using Agrobacterium tumefaciens. Plant Cell Rep 15:980–985
Nadolska-Orczyk A, Orczyk W (2000) Study of the factors influencing Agrobacterium-mediated transformation of pea (Pisum sativum L.). Mol Breed 6:185–194
Prakash NS, Pental D, Bhalla-Sarin N (1994) Regeneration of pigeonpea (Cajanus cajan) from cotyledonary node via multiple shoot formation. Plant Cell Rep 13:623–627
Prasad MG, Sridevi V, Kumar MS (2014) Efficient plant regeneration of green gram (Vigna radiata (L.) Wilczek) via cotyledonary explants. Int J Adv Res 2:55–59
Rao S, Patil P, Kaviraj CP (2005) Callus induction and organogenesis from various explants in Vigna radiata (L.) Wilczek. Indian J Biotechnol 4:556–560
Sagare DB, Mohanty IC (2015) In vitro regeneration system in green gram (Vigna radiata L., Wilczek, cv. Sujata): A recalcitrant legume crop. Res J Agri Sci 6:64–67
Sahoo L, Jaiwal PK (2008) Asiatic beans. In: Kole C, Hall TC (eds) A Compendium of transgenic crop plants. Blackwell Publishing, Oxford, UK. pp 115–132
Saini R, Jaiwal PK (2005) Efficient transformation of a recalcitrant grain legume Vigna mungo L. Hepper via Agrobacterium-mediated gene transfer into shoot apical meristem cultures. Plant Cell Rep 24:164–171
Saini R, Jaiwal PK (2007) Agrobacterium tumefaciens-mediated transformation of blackgram: An assessment of factors influencing the efficiency of uidA gene transfer. Biol Plant 51:69–74
Saini R Jaiwal S, Jaiwal PK (2003) Stable genetic transformation of Vigna mungo L. Hepper via Agrobacterium tumefaciens. Plant Cell Rep 21:851–859
Sangwan RS, Bourgoies Y, Brow S, Vasseur G, Sangwan-Norreel BS (1992) Characterization of competent cells and early events of Agrobacterium-mediated transformation of Arabidopsis thaliana. Planta 188:439–456
Santalla M, Power JB, Davey MR (1998) Efficient in vitro shoot regeneration responses of Phaseolus vulgaris and P. coccineus. Euphytica 102:195–202
Sivakumar P, Gnanam R, Ramakrishnan K, Manickam A (2010) Somatic embryogenesis and regeneration of Vigna radiata. Biol Plant 54:245–251
Somers DA, Somac DA, Olhoft PM (2003) Recent advances in legume transformation. Plant Physiol 131:892–901
Sonia, Saini R, Singh RP, Jaiwal PK (2007) Agrobacterium tumifaciens-mediated transfer of Phaseolus vulgaris α-amylase inhibitor-1 gene into mungbean (Vigna radiata L. Wilczek) using bar as selectable marker. Plant Cell Rep 26:187–198
Srinivasan T, Verma VK and Kirti PB (2004) Efficient shoot regeneration in pigeon pea, Cajanus cajan (L) Millisp using seedling petioles. Curr Sci 86:30–32
Srinivasan T, Kumar KRR, Kirti PB (2010) Establishment of efficient and rapid regeneration system for some diploid wild species of Arachis. Plant Cell Tiss Org Cult 101:303–309
Stachel SE, Messens E, Montagu MV, Zambryski P (1985) Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318:624–629
Stachel SE, Nester EW, Zambryski P (1986) A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proc Natl Acad Sci USA 83:379–383
Tripathi L, Singh AK, Singh S, Singh R, Chaudhary S, Sanyal I, Amla DV (2013) Optimization of regeneration and Agrobacterium-mediated transformation of immature cotyledons of chickpea (Cicer arietinum L.). Plant Cell Tiss Organ Cult 113:513–527
Varshney RK, Close TJ, Singh NK, Hoisington DA, Cook DR (2009) Orphan legumes enter the genomics era. Curr Opin Plant Biol 12:202–210
Vijayan S, Kirti PB (2012) Mungbean plants expressing BjNPR1 exhibit enhanced resistance against the seedling rot pathogen, Rhizoctonia solani. Transgenic Res 21:193–200
Vijayan S, Beena MR, Kirti PB (2006) Effective and simple regeneration of mung bean (Vigna radaia (L.) Wilczek) using cotyledonary node explants. J Plant Biochem Biotechnol 15:131–134
Yadav SK, Krishna MG, Maheswari M, Vanaja M, Venkateswarlu B (2010a) High frequency induction of multiple shoots and plant regeneration from cotyledonary nodal explant of mung bean (Vigna radiata (L.) Wilczek). J Plant Biochem Biotech 19:267–270
Yadav SK, Sreenu P, Maheswari M, Vanaja M, Venkateswarlu B (2010b) Efficient shoot regeneration from double cotyledonary node explants of green gram (Vigna radiata (L.) Wilczek). Indian J Biotechnol 9:403–407
Yadav SK, Katikala S, Yellisetty V, Kannepalle A, Narayana JL, Maddi V, Mandapaka M, Shanker AK, Bandi V and Bharadwaja PK (2012) Optimization of Agrobacterium-mediated genetic transformation of cotyledonary node explants of Vigna radiata. SpringerPlus 1:59–67
Acknowledgments
Author GKM is greatly thankful to Agri Biotech Foundation for providing facilities to conduct part of these studies.
Authors contributions
GKM contributed to the acquisition of data by conducting different experiments and manuscript preparation. VNJ has made substantial contribution in critically drafting and editing the manuscript. GM contributed by giving valuable suggestions in preparation of manuscript. PBK has helped in initial concept design and generously provided the AnnBj1 gene construct. SKY conceived the study and contributed to designing experiments, data analysis and critical revision of manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mekala, G.K., Juturu, V.N., Mallikarjuna, G. et al. Optimization of Agrobacterium-mediated genetic transformation of shoot tip explants of green gram (Vigna radiata (L.) Wilczek). Plant Cell Tiss Organ Cult 127, 651–663 (2016). https://doi.org/10.1007/s11240-016-1085-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1085-3