Abstract
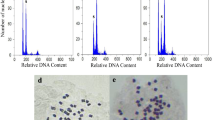
The induction of polyploids in loquat (Eriobotrya japonica (Thunb.) Lindl.) is of great interest for producing larger and seedless fruits according to market demands. Under this premise, this work was aimed to obtain tetraploid plants of loquat using the antimitotic agent colchicine. Experiments consisted in applying colchicine on shoot apex from in vitro-grown plants, in vitro-grown whole plants and ungerminated seeds. Treatments on the shoot apex or submerging whole plants produced no stable polyploids. Conversely, subjecting ungerminated seeds to colchicine produced two triploids in the 0.5 % (w/v) solution after 24 h and one tetraploid after 48 h. The triploids obtained among treated seeds make us believe that these plants were present in the hybrids original seedlot. Polyploidy levels were firstly detected by flow cytometry and later confirmed by chromosome counting and morphological characteristics. The relative fluorescence was 1.5-fold higher in triploids and twofold higher in tetraploids as compared to diploids. As expected, the chromosome number was 2n = 34 in diploids, 2n = 51 in triploids and 2n = 68 in the tetraploid. Moreover, differences in morphological characteristics between diploid and polyploid plants were significant. The tetraploid plant was more compact than triploids or diploids. Particularly, stomata of polyploids were larger with lower density than diploids. Results indicate that induction of polyploidy in loquat species is a reliable tool for breeding new loquat varieties.





Similar content being viewed by others
References
Aleza P, Juarez J, Ollitrault P, Navarro L (2009) Production of tetraploid plants of non apomictic citrus genotypes. Plant Cell Rep 28:1837–1846. doi:10.1007/s00299-009-0783-2
Aryavand A, Ehdaie B, Tran B, Waines JG (2003) Stomatal frequency and size differentiate ploidy levels in Aegilops neglecta. Genet Resour Crop Ev 50:175–182. doi:10.1023/A:1022941532372
Badenes ML, Janick J, Shunquan L, Zhike Z, Guolo LL, Weixing W (2013) Breeding loquat. In: Janick J (ed) Plant breeding reviews, vol 37. John Wiley & Sons, Inc., NJ, USA, pp 259–296. doi:10.1002/9781118497869.ch5
Blakeslee AF, Avery AG (1937) Methods of inducing chromosome doubling in plants by treatment with colchicine. Science 86:408 abstract 5
Blasco M, Naval MM, Zuriaga E, Badenes ML (2014) Genetic variation and diversity among loquat accessions. Tree Genet Genomes. doi:10.1007/s11295-014-0768-3
Bouvier L, Fillon FR, Lespinasse Y (1994) Oryzalin as an efficient agent for chromosome doubling of haploid apple shoots in vitro. Plant Breed 113(4):343–346. doi:10.1111/j.1439-0523.1994.tb00748.x
D’Hont A, Grivet L, Feldmann P, Rao P, Berding N, Glaszmann JC (1996) Characterisation of the double genome structure of modern sugarcane cultivars (Saccharum spp.) by molecular cytogenetics. Mol Gen Genet 250:405–413. doi:10.1007/BF02174028
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plants tissue in vitro. Plant Cell Tiss Org 104:359–373. doi:10.1007/s11240-010-9786-5
Doležel J (1997) Application of flow cytometry for the study of plant genomes. J Appl Genet 38:285–302
Einset J, Imhofe B (1951) Chromosome numbers of apple varieties and sports, 3. Proc Am Soc Hortic Sci 58:103–108
Gamiette F, Bakry F, Ano G (1999) Ploidy determination of some yam species (Dioscorea spp.) by flow cytometry and conventional chromosomes counting. Genet Resour Crop Evol 46:19–27. doi:10.1023/A:1008649130567
Gisbert AD, Gillem A, Martínez-Calvo J, Llácer G, Badenes ML (2006) Contribution of biotechnology in genetic studies and breeding of loquat. Acta Hortic 750:93–96
Gisbert AD, Romero C, Martínez-Calvo JM, Leida C, Llácer G, Badenes ML (2009) Genetic diversity evaluation of a loquat (Eriobotrya japonica (Thunb) Lindl) germplasm collection by SSRs and S-allele fragments. Euphytica 168(1):121–134. doi:10.1007/s10681-009-9901-z
Guo QG, Li XL, Wang WX, He Q, Liang GL (2007) Occurrence of natural triploids in loquat. In: Proceedings of the second international symposium on Loquat, Guangzhou, China, pp 128–128. ISBN 978-90-66055-40-7
Hahn SK, Bai KV, Asiedu R (1990) Tetraploids, triploids and 2n pollen from diploid interspecific crosses with cassava. Theor Appl Genet 79:433–439. doi:10.1007/BF00226148
Harbard JL, Griffin AR, Foster S, Brooker C, Kha LD, Koutoulis A (2012) Production of colchicine induced autotetraploids as a basis for sterility breeding in A. mangium Willd. Forestry 85:427–436. doi:10.1093/forestry/cps041
He Q, Wang W, Guo Q, Xiang S, Li X, Liang G (2012) Genetic diversity and utilization of triploid loquats (E. japonica Lindl). In: Caliskan M (ed) Genetic diversity in plants, pp 197–208. ISBN 978-953-51-0185-7, Published: March 14, 2012 under CC BY 3.0 license. doi:10.5772/34155
Kagan-Zur V, Yaron-Miron D, Mizrahi Y (1991) A study of triploid tomato fruit attributes. J Am Soc Hortic Sci 116(2):228–231
Khazaei H, Monneveux P, Hongbo S, Mohammady S (2010) Variation for stomatal characteristics and water use efficiency among diploid, tetraploid and hexaploid Iranian wheat landraces. Genet Resour Crop Evol 57:307. doi:10.1007/s10722-009-9471-x
Kihara H (1951) Triploid watermelons. Proc Am Soc Hortic Sci 58:217–230
Kulkarni M, Borse T (2010) Induced polyploidy with gigas expression for root traits in Capsicum annum (L.). Plant Breed 129:461–464. doi:10.1111/j.1439-0523.2009.01696.x
Lehrer JM, Brand MH, Lubell JD (2008) Induction of tetraploidy in meristematically active seeds of Japanese barberry (Berberis thunbergii var. atropurpurea) through exposure to colchicine and oryzalin. Sci Hortic Amst 119:67–71. doi:10.1016/j.scienta.2008.07.003
Liu G, Li Z, Bao M (2007) Colchicine-induced chromosome doubling in Platanus acerifolia and its effect on plant morphology. Euphytica 157:145–154. doi:10.1007/s10681-007-9406-6
Liu P, Zhao ZH, Dai L, Liu XY, Peng JY, Peng SQ, Zhou ZJ (2009) Genetic variations of Ziziphus cultivar ‘Zanhuangdazao’ by using RAPD technique. Acta Hortic 840:149–154
Loureiro J, Pinto G, Lopes T, Doležel J, Santos C (2005) Assessment of ploidy stability of the somatic embryogenesis process in Quercus suber L. using flow cytometry. Planta 221:815–822. doi:10.1007/s00425-005-1492-x
Mishra MK (1997) Stomatal characteristics at different ploidy levels in Coffea L. Ann Bot Lond 80(5):689–692. doi:10.1006/anbo.1997.0491
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Plant Physiol 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Ollitrault P, Froelicher Y, Dambier D, Luro F, Yamamoto M (2007) Seedlessness and ploidy manipulations. In: Khan IA (ed) Citrus genetics, breeding and biotechnology. CAB International, Wallingford, pp 197–218
Ollitrault P, Dambier D, Luro F, Froelicher Y (2008) Ploidy manipulation for breeding seedless triploid citrus. Plant Breeding Reviews 20:323–354. doi:10.1002/9780470380130.ch7
Pintos B, Manzanera JA, Bueno MA (2007) Antimitotic agents increase the productivity of double-haploid embryos from cork oak anther culture. J Plant Physiol 164:1595–1604
Predieri S (2001) Mutation induction and tissue culture in improving fruits. Plant Cell Tiss Org 64:185–210. doi:10.1023/A:1010623203554
Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29:467–501. doi:10.1146/annurev.ecolsys.29.1.467
Rubuluza T, Nikolova RV, Smith MT, Hannweg K (2007) In vitro induction of tetraploids in Colophospermum mopane by colchicine. South Afr J Bot 77:259–261
Rugini E, Pannelli G, Ceccarelli M, Muganu M (1996) Isolation of triploid and tetraploid olive (Olea europaea L.) plants from mixoploid cv. ‘Frantoio’ and ‘Leccino’ mutants by in vivo and in vitro selection. Plant Breed 115(1):23–27. doi:10.1111/j.1439-0523.1996.tb00865.x
Sakhanokho HF, Rajasekaran K, Kelley RV, Farjdj NI (2009) Induced polyploidy in diploid ornamental ginger (Hedychium muluense R. M. Smith) using colchicine and oryzalin. HortScience 44(7):1809–1814
Sanford JC (1983) Ploidy manipulations. In: Moore JN, Janick J (eds) Methods in fruit breeding. Purdue University Press, West Lafayette, pp 100–123
Schifino MT, Moraes-Fernandes MIB (1987) Induction of polyploidy and cytological characterization of autotetraploids of Trifolium riograndense Burkart (Leguminosae). Euphytica 36:863–872. doi:10.1007/BF00051871
Simmonds NW, Sheperd K (1955) The taxonomy and origins of the cultivated bananas. Bot J Linn Soc 55:302–312. doi:10.1111/j.1095-8339.1955.tb00015.x
Soriano JM, Romero C, Vilanova S, Llácer G, Badenes ML (2005) Genetic diversity of loquat germplasm (Eriobotrya japonica (Thunb) Lindl) assessed by SSR markers. Genome 48(1):108–114
Sun QR, Sun SH, Li LG, Bell RL (2009) In vitro colchicine-induced polyploidy plantlet production and regeneration from leaf explants of the diploid pear (Pyrus communis L.) cultivar ‘Fertility’. J Hortic Sci Biotech 84:548–552
Tel-Zur N, Dudai M, Raveh E, Mizrahi Y (2001) In situ induction of chromosome doubling in vine cacti (Cactaceae). Sci Hortic Amst 129:570–576. doi:10.1016/j.scienta.2011.04.027
Tian S, Li B, Ding Z (2007) Physiological properties and storage technologies of loquat fruit. Fresh Prod 1(1):76–81
Väinölä A (2000) Polyploidization and early screening of Rhododendron hybrids. Euphytica 112:239–244. doi:10.1023/A:1003994800440
Wu J, Ferguson AR, Murray BG, Duffy AM, Jia Y, Cheng C, Martin PJ (2013) Fruit quality in induced polyploids of Actinidia chinensis. HortScience 48(6):701–707
Xing SH, Sun XF, Tang KX et al (2011) Induction and flow cytometry identification of tetraploids from seed-derived explants through colchicine treatments in Catharanthus roseus (L.) G. Don. J Biomed Biotechnol 2011:793198. doi:10.1155/2011/793198
Zhang Q, Luo F, Liu L, Guo F (2010) In vitro induction of tetraploids in crape myrtle (Lagerstroemia indica L.). Plant Cell Tiss Org 101:41–47. doi:10.1007/s11240-009-9660-5
Acknowledgments
We acknowledge Prof. Janick from revision and edition of the MS, and Dr. Jaime Cebolla from UPV and Dr. Carlos Romero from CSIC for their critical reading of this manuscript. This research was funded by the Grant RF 2010-0003-00-00 from the National Agency ‘Instituto Nacional de Investigaciones Agrarias’ (INIA). MBV was funded by a fellowship from INIA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blasco, M., Badenes, M.L. & Naval, M.d.M. Colchicine-induced polyploidy in loquat (Eriobotrya japonica (Thunb.) Lindl.). Plant Cell Tiss Organ Cult 120, 453–461 (2015). https://doi.org/10.1007/s11240-014-0612-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0612-3