Abstract
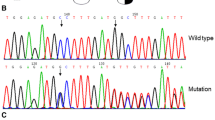
To identify the pathogenesis of a Chinese woman diagnosed with dysfibrinogenemia. A patient from Nanjing presented with a low plasma concentration of fibrinogen and a normal level of antigen of fibrinogen. This abnormality was also detected in her son. To detect whether the genetic mutation was responsible for the dysfibrinogenemia, genomic DNA was extracted and amplified by polymerase chain reaction, and DNA sequencing was performed on the purified PCR products. Restriction fragment length polymorphism (RFLP), molecular modeling and homologous sequences alignment were performed. Two heterozygous missense variants, AαArg16His and γAsp185Asn, were discovered in the proband. Only the former was detected in her son. AαArg16His had been reported by other teams, and γAsp185Asn was identified first in our study as a novel variant. RFLP was performed and indicated that the novel failed to be found in normal subjects. Furthermore, it was suggested to be responsible for dysfibrinogenemia depending on the molecular modeling and homologous sequence alignment. The heterozygous AαArg16His and γAsp185Asn identified in the study probably underlie the dysfibrinogenemia in this pedigree, with the latter being identified for the first time.





Similar content being viewed by others
References
Asselta R, Duga S, Tenchini ML (2006) The molecular basis of quantitative fibrinogen disorders. J Thromb Haemost 4:2115–2129
Spraggon G, Everse SJ, Doolittle RF (1997) Crystal structures of fragment D from human fibrinogen and its crosslinked counterpart from fibrin. Nature 389:455–462
Redman CM, Xia H (2001) Fibrinogen biosynthesis:Assembly, intracellular degradation, and association with lipid synthesis and secretion. Ann NY Acad Sci 936:480–495
Mosesson MW, Siebenlist KR, Meh DA (2001) The structure and biological features of fibrinogen and fibrin. Ann NY Acad Sci 936:11–30
Standeven KF, Ariëns RA, Grant PJ (2005) The molecular physiology and pathology of fibrin structure/function. Blood Rev 19:275–288
Huang DD, Chen HY, Hu XB, Wang X, Wang H (2015) Identification of a novel splicing mutation in the fibrinogen gamma chain gene leading to dysfibrinogenaemia in a Chinese pedigree. Pathology 47:145–150
Mathonnet F, Peltier JY, Roda L, de Raucourt E, D’Hailly F, Tetegan M et al (2001) Three new cases of dysfibrinogenemia Poissy III, Saint-Germain I and Tahiti. Thromb Res 103:201–207
Galanakis DK, Henschen A, Keeling M (2006) Fibrinogen Louisville: an Aα16 Arg→His defect that forms no hybrid molecules in heterozygous individuals and inhibits aggregation of normal fibrin monomers. Ann NY Acad Sci 408:644–648
Kotlín R, Chytilová M, Suttnar J, Riedel T, Salaj P, Blatný J et al (2007) Fibrinogen Novy Jicin and Praha II: cases of hereditary Aα16Arg→Cys and Aα16Arg→His dysfibrinogenemia. Thromb Res 121:75–84
Meh D, Siebenlist KR, Mosesson MW (1996) Identification and characterization of the thrombin binding sites on fibrin. J Biol Chem 271:23121–23125
Everse SJ, Spraggon G, Veerapandian L, Riley M, Doolittle RF (1998) Crystal structure of fragment double-D from human fibrin with two different bound ligands. BioChemistry 37:8637–8642
Sugo T, Endo H, Matsuda M, Ohmori T, Madoiwa S, Mimuro J et al (2006) A classification of the fibrin network structures formed from the hereditary dysfibrinogens. J Thromb Haemost 4:1738–1746
Asselta R, Robusto M, Plate M, Santoro C, Peyvandi F, Duga S (2015) Molecular characterization of 7 patients affected by dys- or hypo-dysfibrinogenemia: identification of a novel mutation in the fibrinogen Bbeta chain causing a gain of glycosylation. Thromb Res 136:168–174
Lam PV, Goldman R, Karagiannis K, Narsule T, Simonyan V, Soika V et al (2013) Structure-based comparative analysis and prediction of N-linked glycosylation sites in evolutionarily distant eukaryotes. Genomics Proteomics Bioinform 11:96–104
Yakovlev S, Litvinovich S, Loukinov D, Medved L (2000) Role of the β-strand insert in the central domain of the fibrinogen γ-module. BioChemistry 39:15721–15729
Acknowledgements
This work was supported partly by the National Natural Science Foundation of China (81400162, 81570174), Clinical Medical Special Foundation of Jiangsu Province (BL20122005), and Medical Science and Technology Development Foundation of Nanjing Department of Health (ZKM06052, YKK08065).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Zhou, N., Xu, P., Zhou, M. et al. A novel fibrinogen variant: dysfibrinogenemia associated with γAsp185Asn substitution. J Thromb Thrombolysis 44, 139–144 (2017). https://doi.org/10.1007/s11239-017-1496-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-017-1496-y