Abstract
Greater use of evidence-based therapies has improved outcomes for patients with acute coronary syndromes (ACS) in recent decades. Consequently, more ACS patients are surviving beyond 12 months; however, limited data exist to guide treatment in these patients. Long-term outcomes have not improved in non-ST-segment elevation myocardial infarction (NSTEMI) patients at the same rate seen in ST-segment elevation myocardial infarction patients, possibly reflecting NSTEMI patients’ more complex clinical phenotype, including older age, greater burden of comorbidities and higher likelihood of a previous myocardial infarction (MI). This complexity impacts clinical decision-making, particularly in high-risk NSTEMI patients, in whom risk–benefit assessments are problematical. This review examines the need for more effective long-term management of NSTEMI patients who survive ≥12 months after MI. Ongoing risk assessment using objective measures of risk (for bleeding and ischemia) should be used in all post-MI patients. While 12 months appears to be the optimal duration of dual antiplatelet therapy for most patients, this may not be the case for high-risk patients, and more research is urgently needed in this population. A recent subgroup analysis from the DAPT study in patients with or without MI who had undergone coronary stenting (31 % presented with MI; 53 % had NSTEMI) and the prospective PEGASUS-TIMI 54 trial in patients with a prior MI and at least one other risk factor (40 % had NSTEMI) demonstrated that long-term dual antiplatelet therapy improved cardiovascular outcomes but increased bleeding. Further studies will help clarify the role of dual antiplatelet therapy in stable post-NSTEMI patients.
Similar content being viewed by others
Introduction
Approximately 50–75 % of patients experiencing an acute coronary event in the US each year have a non-ST-segment elevation myocardial infarction (NSTEMI) [1–3], and the proportion of NSTEMI events is increasing [4]. Mortality rates after myocardial infarction (MI) have decreased over the last 20 years [3], but improvement in outcomes differs between ST-segment elevation myocardial infarction (STEMI) and NSTEMI patients. Compared with STEMI patients, NSTEMI patients have lower short-term mortality rates, and higher rates of long-term mortality, even after adjustment for risk factors [2, 4]. One-year mortality rates for STEMI patients have declined recently, but in NSTEMI patients, the trend is inconsistent and less marked [3]. Registry data suggest that the 10-year survival rate after NSTEMI is around 50 % [5].
Several years ago, the only interventional options for acute coronary syndromes (ACS) were balloon angioplasty or surgery, and post-percutaneous coronary intervention (PCI) patients received warfarin. PCI provided only moderate benefit [6], but the advent of coronary stents and more potent antiplatelet agents have significantly improved outcomes, such that PCI is now recommended for most NSTEMI patients [7, 8]. Evidence-based therapy use, during and after hospitalization, has increased over the past several years in both STEMI and NSTEMI patients [3]. Yet, despite this increase and the associated improvement in outcomes after ACS, a significant residual risk, in the short (up to 12 months) and long term (≥3 years or longer), for cardiovascular-related death remains [9].
A key question is why are long-term outcomes not improving in NSTEMI patients as for STEMI patients? The likely reason is that patients with NSTEMI tend to have a more complex clinical phenotype. Compared with STEMI patients, NSTEMI patients tend to be older, have more comorbidities, are more likely to have an MI history, and to experience recurrent ischemia after the acute event [2, 3]. Thus, clinical decision making is more complicated, particularly in high-risk NSTEMI patients, as risk–benefit assessment is less straightforward. Poorer outcomes in high-risk NSTEMI populations indicate the need for more effective treatments. Clear evidence supports treatment decisions in the acute and post-acute phase of ACS; however, data are limited to guide long-term management in the growing population of patients who survive beyond 12 months after an event– many of whom are elderly and have comorbidities.
This review examines the need for more effective management; reviews the evidence and rationale for treatment; identifies opportunities to improve outcomes; and outlines recent research addressing unmet needs in high-risk NSTEMI patients. Herein, ‘long term’ refers to outcomes occurring ≥12 months after the initial ACS.
Current treatment recommendations for high-risk patients
An urgent invasive strategy is generally preferred as initial management in NSTEMI patients with refractory angina, signs or symptoms of heart failure, and hemodynamic instability [7]. Patients should undergo angiography within 2 h of admission, with appropriate anti-ischemic, antiplatelet, and anticoagulant therapy [7]. Urgent catheterization is preferred in high-risk patients, who show better outcomes with this approach versus delayed catheterization [10]. Other high-risk NSTEMI patients should undergo an early invasive strategy (within 24 h of presentation) [7]. For in-hospital NSTEMI management, currently recommended dual antiplatelet therapy incorporates aspirin with either clopidogrel or ticagrelor; ticagrelor is preferred in patients undergoing early invasive or ischemia-guided therapy [7].
Previously, the American Heart Association/American College of Cardiology (AHA/ACC) guidelines suggested that any P2Y12 inhibitor could be considered in NSTEMI patients, but the 2014 update [7] brings antiplatelet recommendations more in line with European guidelines. European guidelines recommend ticagrelor for all patients at moderate to high risk of ischemic events, regardless of initial treatment strategy [8]. In Europe, prasugrel is recommended for P2Y12 inhibitor-naïve patients with known coronary anatomy who are proceeding to PCI, unless contraindicated, or patients at high risk of bleeding [8]. Clopidogrel is recommended only for patients who cannot receive ticagrelor or prasugrel [8].
The 2014 AHA/ACC NSTEMI guidelines emphasize secondary prevention, including ongoing use of dual antiplatelet therapy for post-hospital care [7]. Table 1 summarizes the AHA/ACC recommendations for maintenance dosing of antiplatelet agents. As with US guidelines, European guidelines recommend treatment with a P2Y12 inhibitor for at least 12 months after the event [8].
All post-NSTEMI patients should receive beta-blockers and statins long term, unless contraindicated [7]; patients may also require medications to modify risk factors, such as antihypertensive medications to achieve target blood pressure, angiotensin-converting enzyme (ACE) inhibitors for left ventricular dysfunction, and antihyperglycemic agents to maintain HbA1c <7 % [7, 11].
Data supporting current recommendations
CHARISMA assessed clopidogrel plus aspirin in a high-risk cohort of patients with established atherothrombotic disease or at high risk of atherothrombosis [12]. Although the group with risk factors did not necessarily derive clinical benefit from dual antiplatelet therapy, those with established disease did [12]. Therefore, a subanalysis of the high-risk secondary prevention population was undertaken [13]. These patients had prior MI (n = 3846), stroke (n = 3245), or symptomatic peripheral arterial disease (PAD) (n = 2838), and received clopidogrel or placebo, plus aspirin, for a median of 27.6 months. Patients taking clopidogrel plus aspirin had a significantly lower risk of cardiovascular death, MI, or stroke (primary end point) versus those receiving placebo plus aspirin (7.3 vs. 8.8 %; hazard ratio [HR], 0.83 [95 % confidence interval (CI), 0.72–0.96]; p = 0.01). In prior MI cohort, the primary composite end point occurred in 6.6 % of patients taking clopidogrel plus aspirin versus 8.3 % of those taking placebo plus aspirin (HR, 0.774 [95 % CI, 0.613–0.978]; p = 0.031). The benefit of dual antiplatelet therapy was not seen in patients with established coronary artery disease (CAD) without prior MI (Fig. 1) [13], implying that dual antiplatelet therapy may provide a benefit in the post-MI setting, even if initiated some time after the event.
Kaplan–Meier curves for the primary composite end point of cardiovascular death, MI, or stroke in subgroups of patients. a Patients with prior MI in CHARISMA [13]; b Patients with prior MI in the TRA2°P-TIMI 50 trial [9]; c Patients with established coronary artery disease who had not had an MI in CHARISMA [13]. ASA aspirin, CI confidence interval, HR hazard ratio Panels a + c are reprinted from J Am Coll Cardiol 49 (19), Bhatt DL et al. ‘Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial.’ 1982–1988, copyright (2007), with permission from Elsevier. Panel b is reprinted from The Lancet 380, Scirica BM et al. ‘Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA2°P-TIMI 50 trial.’ 1317–1324, copyright (2012) with permission from Elsevier
Current recommendations do not provide clear clinical guidance on the duration of dual antiplatelet therapy, or whether the therapy duration depends on the patient’s risk profile. Previous studies (including two randomized controlled trials) showed no significant benefit in continuing dual antiplatelet therapy beyond 12 months in patients who have undergone PCI and survive event-free for 1 year [14–16]. A meta-analysis of the randomized data showed the overall odds ratio for interrupting dual antiplatelet therapy at 12 months versus continuing therapy was 1.18 (95 % CI, 0.61–2.29 [p = 0.62]) [15]. However, these studies were underpowered, and did not necessarily enroll high-risk patients [14, 15], so the question of whether or not extended treatment would be beneficial in high-risk patients remains unanswered.
Impact of risk factors on outcomes
Optimal, evidence-based treatment after NSTEMI can only reduce the risk of an event. Even optimally treated patients face a residual risk of adverse cardiovascular outcomes due to the underlying disease process, their general health, and any comorbid conditions. So what constitutes ‘high risk’ after NSTEMI?
In the last decade, much has been learned about long-term risk from registry studies, including GRACE, ACTION, GTWG database, and CRUSADE registry data of NSTEMI patients. These registries have contributed to the development of a number of risk assessment tools (see below). A consistent finding is that risk of adverse outcomes increases with older age, male gender, diabetes, worse renal function, renal failure, anemia, prior vascular disease/CAD, heart failure (past or present), poor hemodynamics at presentation, and clinical instability during ACS [17–20].
The Worcester Heart Attack Study (WHAS) investigated long-term risk factors for mortality after discharge specifically in NSTEMI patients, and found that older age, male gender, longer hospital stay, history of stroke, heart failure, or diabetes, and stroke or heart failure during hospitalization all predicted long-term mortality [2]. Patients were studied for up to 5 years after the index event, but analyses did not distinguish between risk factors for death ≤12 versus >12 months [2].
Risk factors for 10-year mortality after NSTEMI in the PRAIS-UK registry were age, ST depression or bundle branch block on initial electrocardiogram (ECG), and a history of heart failure [5]. However, this analysis was based on UK NSTEMI patients in 1998 and 1999, and may not be applicable to a contemporary NSTEMI population either in the UK or elsewhere. EPICOR developed a risk score for mortality in patients with STEMI (n = 4943) and non-ST-elevation ACS (n = 5625) [21]. Twelve independent predictors of mortality were identified. In order of importance, these were: age, lower ejection fraction, poorer EQ-5D quality of life, elevated serum creatinine, in-hospital cardiac complications, chronic obstructive pulmonary disease, elevated blood glucose, male gender, no PCI/coronary artery bypass grafting (CABG) after NSTE-ACS, low hemoglobin, PAD, and on diuretics at discharge. However, the risk score was based on 12-month mortality risk and may not be applicable to long-term outcomes.
Evidence suggests that biomarkers may be useful to help identify high-risk patients after NSTEMI. Recent research identified a number of biomarkers that enhance the accuracy of the GRACE risk assessment in NSTEMI patients, including B-type natriuretic peptide [22], C-terminal vasopressin or copeptin [23], and growth differentiation factor-15 [24]. However, none of these biomarkers has yet been adopted for risk assessment during clinical practice.
In summary, patients at high risk after NSTEMI are likely to be of older age, men, and have cardiovascular (e.g. heart failure, stroke) and non-cardiovascular (e.g. poor renal function, diabetes) comorbidities, and a poorer quality of life than those at low risk.
Determination of risk
Several studies indicate that when established risk assessment methods are not used, physicians tend to underestimate risk in high-risk patients and overestimate risk in low-risk patients [25]. Additionally, physicians tend to estimate risk based on the intensity of treatment received during the ACS [25, 26]. In particular, physicians underestimate risk associated with age, and may view younger ACS patients as having a more aggressive disease phenotype than older patients, while underestimating the impact of age-associated accumulated coronary artery damage [26]. Therefore, it is important that physicians use validated objective measures of risk when assessing ACS patients.
Ischemic risk
Several risk-scoring tools evaluated the risk of subsequent events in ACS patients, some of which can be used in NSTEMI patients (Table 2) [17–19, 27–30]. These risk scores, derived mainly from randomized controlled trials and registry data, assess a patient’s short- to medium-term risk of an adverse outcome (usually death and/or nonfatal MI).
While no risk tool is clearly superior to another, an analysis by the UK National Institute for Health and Clinical Excellence (NICE) suggests that the PURSUIT, GRACE, and PREDICT tools provide better discrimination of mortality risk than the Thrombolysis in Myocardial Infarction (TIMI) score [31]. TIMI and GRACE risk scores are most commonly used, and available as online calculators to simplify risk stratification in clinical practice [32].
Few of these tools were designed to assess long-term risk (past 1 year). However, the GRACE score was a useful predictor of death at 5 years in a mixed ACS population in UK and Belgian GRACE registries [33], and at 10 years in an NSTEMI cohort from the PRAIS UK registry [5].
The PREDICT tool was one of the few designed to predict long-term outcomes, and has a better predictive power for 2- or 6-year outcomes than for 30-day outcomes [17]. However, PREDICT was developed in a mostly white population, and is not specific for NSTEMI patients.
The SYNERGY tool was designed to assess 1-year outcomes in patients surviving 30 days after the acute event [30]. This tool may be particularly useful for the care of long-term, post-hospitalization NSTEMI patients because it excludes risk factors that predict death during the immediate post-ACS period.
Bleeding risk
Few tools exist to assess bleeding risk; the most widely used was developed from the CRUSADE registry data of NSTEMI patients, and is designed to assess risk of in-hospital bleeding. The CRUSADE bleeding risk assessment tool assigns a score based on the patient’s baseline hematocrit, creatinine clearance, heart rate, gender, systolic blood pressure, and presence of prior vascular disease, congestive heart failure on presentation or diabetes mellitus [34].
Another bleeding risk assessment tool was developed from the ACTION-GTWG database [35], including STEMI and NSTEMI patients. The ACTION-GTWG bleeding-risk score is more complicated than CRUSADE, and includes 12 variables. It includes all of the variables in the CRUSADE risk tool (notwithstanding using hemoglobin instead of hematocrit as a measure of anemia, and serum creatinine instead of creatinine clearance for renal function), and also includes body weight, warfarin use, and the presence and type of ST changes on ECG [35]. This tool has been validated for the prediction of major bleeding during hospitalization, but no data are available on its use to predict long-term bleeding.
Balancing the risk of ischemic versus bleeding events
Treatment selection in clinical practice must balance risk of ischemic events with bleeding risk, which is difficult in patients with multiple risk factors. Many risk factors for ischemic events are the same as for bleeding events, complicating decision making. Bleeding during hospitalization for NSTEMI is associated with a higher rate of mortality in the first 30 days, 1 and 3 years after an event, particularly in patients undergoing PCI [36]. This finding may be partly explained by reduced use of dual antiplatelet therapy at discharge in patients with a bleeding event during hospitalization [36].
While bleeding may be a marker for a poor long-term outcome, bleeding may not be causally related to outcome [37]. In an analysis of CHARISMA (overall cohort) comparing patients who continued dual antiplatelet therapy and those who discontinued, the rate of adverse outcomes, both cardiovascular and bleeding events, over 28 months was higher in those who discontinued versus continued antiplatelet therapy [38]. The increased bleeding rate among patients discontinuing dual antiplatelet therapy was ascribed to patients who discontinued being more likely to have ischemic risk factors, such as age and history of significant cardiovascular disease; additionally, therapy may have been discontinued because of prior bleeding [38].
For many high-risk patients, the risk-benefit profile is not clear-cut, and should be considered on an individual basis. For example, triple therapy (dual antiplatelet therapy plus an oral anticoagulant) has been evaluated in ACS patients. The ATLAS-ACS 2 TIMI-51 trial evaluated rivaroxaban (a selective factor Xa inhibitor) versus placebo in ACS patients; all patients received aspirin plus a thienopyridine [39]. Rivaroxaban reduced the rate of the composite end point of MI, stroke, or death from cardiovascular causes versus placebo (8.9 vs. 10.7 %; HR, 0.84 [95 % CI, 0.74–0.96]; p = 0.008). However, versus placebo, rivaroxaban also increased rates of major bleeding not related to CABG (2.1 vs. 0.6 %, p < 0.001) and intracranial hemorrhage (0.6 vs. 0.2 %, p = 0.009), without a significant increase in fatal bleeding (0.3 vs. 0.2 %, p = 0.66) [39]. These data suggest that, in some patients, the increased risk of major bleeding associated with triple therapy may outweigh the benefit associated with a reduction in ischemic events [39], reinforcing the importance of careful risk assessment in each patient based on clinical and demographic characteristics.
Are patients being treated on the basis of risk?
Early data from the CRUSADE registry showed that dual antiplatelet therapy was underutilized at discharge in NSTEMI patients, and underutilization was greater in some patient subgroups—those not undergoing PCI, those aged >75 years, women, and Hispanic patients [40]. Fewer than 50 % of eligible NSTEMI patients not undergoing PCI received dual antiplatelet therapy in the CRUSADE registry in 2002/2003. This observation was also true in the ACTION registry (2007–2010), which showed that 40.7 % of NSTEMI patients in the US not undergoing PCI received dual antiplatelet therapy [41]. This proportion is lower than in NSTEMI patients not undergoing PCI reported in the UK or Swedish registries during the same period (70.6 and 48.8 %, respectively) [41]. However, the overall rate of discharge antiplatelet use among NSTEMI patients reported in the ACTION registry was around 74 % between 2009 and 2012 [42].
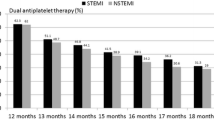
An interesting finding in the recent analysis of ACTION registry data (2009–2012) is the use of prasugrel in patients in whom it is not indicated, or should be used with caution. Prasugrel was used in 2 % of medically managed patients, 2 % of patients aged ≥75 years, and 5 % of patients weighing <60 kg [42]. In addition, the highest rate of prasugrel use was in patients with the lowest risk of ischemic or bleeding events (Fig. 2) [42], despite the fact that evidence supports its use in individuals at high risk of ischemic events.
Prasugrel use by mortality and bleeding risk in the NSTEMI population of the ACTION registry [42]. Reproduced from Sherwood MW et al. ‘Early clopidogrel versus prasugrel use among contemporary STEMI and NSTEMI patients in the US: insights from the National Cardiovascular Data Registry. J Am Heart Assoc 2014;3:e000849, with permission from Wiley. ©2014 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell
Collectively, these data suggest that high-risk patients may be undertreated after NSTEMI. Just as clinicians tend to underestimate the ischemic risk in high-risk patients [25], it is possible that they may be overestimating the risk of bleeding, as has been shown with the use of antithrombotic therapy in high-risk atrial fibrillation patients [43]. Minimizing the risk of bleeding is important, but undertreating may increase the risk of ischemic events. Ischemia has irreversible effects on tissue and may have long-term sequelae (e.g. heart failure, stroke-related disability), whereas bleeding can almost always be controlled and, with the exception of intracerebral bleeding, does not generally have long-lasting effects.
Treatment options: current knowledge and future research
As more patients survive ACS, more evidence is needed to support long-term treatment decisions, but few studies have investigated outcomes and strategies for ≥12 months after an event. The TRA2°P-TIMI 50 trial investigated the effect of the protease-activated receptor (PAR)-1 antagonist voraxapar versus placebo on cardiovascular outcomes (composite end point: cardiovascular death, MI, or stroke) in patients with a history of atherothrombosis [44]. The patient cohort included a large subgroup with a history of MI (n = 17 779), but these patients had the qualifying MI between 2 weeks and 12 months prior to enrolment [9]. In these patients with a history of MI (all of whom were taking aspirin), voraxapar significantly reduced the 3-year risk of the composite end point by 20 %, compared with placebo (HR, 0.80 [95 % CI, 0.72–0.89]; p < 0.0001), but at the expense of a significant increase in the risk of moderate or severe bleeding (HR, 1.61 [95 % CI, 1.31–1.97]; p < 0.0001) [9]. When reviewed alongside the data from CHARISMA, these data suggest that dual antiplatelet therapy may be an effective strategy in stable post-MI patients, and provides continued risk reduction when continued for longer than 12 months (Fig. 1). However, the data are not specific to NSTEMI patients and provide little guidance on the effect of treatment started ≥12 months after MI.
The APOLLO study uses electronic medical record data from patients who survive the first 12 months after an ACS to evaluate their subsequent outcomes. This large-scale study is being conducted in Sweden, England, France, and the US, and will provide important information about outcomes in a ‘real-world,’ unselected patient cohort representative of clinical practice. Preliminary data suggested that in the US, outcome rates over 3 years were worse than those in the studied European countries. For example, the all-cause mortality rate over 3 years was 30.2 % in the US, compared with 20.1 % in Sweden, 14.3 % in France, and 13.7 % in the UK [45]. However, US patients also had more comorbidities than patients in other countries, and the difference was less marked (although significant vs. Sweden and UK) after adjustment for risk factors; adjusted all-cause mortality was 12.8 % in the US, compared with 12.4 % in France, 11.2 % in Sweden, and 8.7 % in the UK [45]. The DAPT study investigated the incidence of stent thrombosis and major cardiovascular and cerebrovascular events (a composite of death, MI, or stroke) in patients who had received a stent [46]. In this randomized controlled trial, 9961 patients received thienopyridine therapy (clopidogrel or prasugrel) for 12 months, and were then randomly assigned to either continue thienopyridine therapy or receive placebo for 18 months. Rates of stent thrombosis in the thienopyridine group were reduced versus placebo (0.4 vs. 1.4 %; HR, 0.29 [95 % CI, 0.17–0.48]; p < 0.001), as were composite end-point events (4.3 vs. 5.9 %; HR, 0.71 [95 % CI, 0.59–0.85]; p < 0.001) and MI (2.1 vs. 4.1 %; HR, 0.47; p < 0.001). However, all-cause mortality was higher in the group that continued thienopyridine treatment, compared with placebo (2 vs. 1.5 %; HR, 1.36 [95 % CI, 1.00–1.85]; p = 0.05). The primary safety end point (rate of moderate or severe bleeding) was higher in the thienopyridine group versus placebo (2.5 vs. 1.6 %, p = 0.001). A recent subgroup analysis from the DAPT study examined these same efficacy and safety end points among patients undergoing coronary stenting after presentation with or without an acute MI (n = 3576 presented with MI [31 %]; 53 % had NSTEMI) [47]. Compared with placebo, long-term thienopyridine therapy statistically significantly reduced the occurrence of stent thrombosis in both patient subgroups and significantly reduced major adverse cardiovascular and cerebrovascular events to a greater degree in the MI group, but with a significantly higher occurrence of bleeding in both subgroups (Table 3).
The potential for shortening the duration of dual anti-platelet therapy in patients following drug-eluting stent-PCI was evaluated in the ISAR-SAFE trial [48]. The trial results showed that the primary composite end point (death, MI, stent thrombosis, stroke or TIMI major bleeding) did not differ between the group treated for 6 versus 12 months (1.5 vs. 1.6 %, Δ −0.1 %, [1-sided 95 % CI, 0.5 %], P noninferiority < 0.001). A trend toward lower rates of bleeding was observed in the group receiving 6 versus 12 months of dual antiplatelet therapy. Similar findings were observed in the ITALIC/ITALIC+ trial, which reported that for patients who respond well to aspirin, 6 months is non-inferior to 24 months of dual antiplatelet therapy for the composite primary end point of death, MI, target lesion revascularization, stroke, and major bleeding [49].
The multinational PEGASUS-TIMI 54 trial prospectively investigated the effect of aspirin and ticagrelor on outcomes in patients who had an MI 1–3 years previously [50, 51]. This trial included 21,162 high-risk patients, all currently taking low-dose aspirin (75–150 mg/day). As well as a history of MI, patients had at least one of the following risk factors: age ≥65 years, diabetes, a second prior MI, multivessel CAD that included ≥50 % occlusion in 2 or more coronary arteries, or chronic renal dysfunction. Patients were randomized 1:1:1 to placebo, ticagrelor 90 mg twice daily, or ticagrelor 60 mg twice daily. Median follow-up was 33 months. Both ticagrelor doses significantly reduced the primary efficacy end point (composite of cardiovascular death, MI, or stroke), compared with placebo. At 3 years, the Kaplan–Meier rates were 7.85 % (ticagrelor 90 mg), 7.77 % (ticagrelor 60 mg), and 9.04 % (placebo); ticagrelor 90 mg: HR, 0.85 (95 % CI, 0.75–0.96]; p = 0.008; ticagrelor 60 mg: HR, 0.84 (95 % CI, 0.74–0.95]; p = 0.004. Rates of TIMI major bleeding (primary safety end point) were higher with ticagrelor (90 mg: 2.60 %; 60 mg: 2.30 %) versus placebo (1.06 %; p < 0.001 for each ticagrelor dose) [50]. The PEGASUS-TIMI 54 data demonstrate the potential benefit of dual antiplatelet therapy (ticagrelor and aspirin) beyond 12 months in high-risk, post-MI patients. Although this trial was not specific for patients with NSTEMI, at baseline, 40.3 % of the overall patients had NSTEMI.
Of the five randomized studies evaluating prolonged (>12 months) dual antiplatelet therapy, four studies (including one subgroup analysis) demonstrated significant clinical benefit (reduction in the primary efficacy end point) with extending treatment, compared with controls. Although in four studies, prolonged treatment resulted in increased bleeding (Table 3). None of these trials were specific for patients with NSTEMI, thus highlighting the need for further research. Subanalyses of NSTEMI patients in PEGASUS-TIMI 54, and more prospective studies in high-risk NSTEMI patients, will advance our understanding of long-term management of these patients.
Conclusions
There are currently limited data to guide clinical decision making around optimal secondary preventive therapies in NSTEMI patients who survive 12 months or more after MI. While 12 months appears to be the optimal duration of dual antiplatelet therapy for most patients, this may not be the case for high-risk patients. Ongoing risk assessment (for bleeding and ischemia) is important in all post-MI patients, and clinicians should use objective measures of assessment whenever possible to avoid over- or under-estimating future risk. Physicians also need to regularly assess the risks and benefits of all therapies to suit the patient’s clinical status, which may change over time in the years following ACS. More research is urgently needed to help guide therapeutic decision making during long-term management of complex patients after NSTEMI.
References
Mozaffarian D, Benjamin EJ, Go AS et al (2015) Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131:e29–e322
Darling CE, Fisher KA, McManus DD et al (2013) Survival after hospital discharge for ST-segment elevation and non-ST-segment elevation acute myocardial infarction: a population-based study. Clin Epidemiol 5:229–236
McManus DD, Gore J, Yarzebski J et al (2011) Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med 124:40–47
Chan MY, Sun JL, Newby K et al (2009) Long-term mortality of patients undergoing cardiac catheterization for ST-elevation and non-ST-elevation myocardial infarction. Circulation 119:3110–3117
Erdem G, Bakhai A, Taneja AK et al (2013) Rates and causes of death from non-ST elevation acute coronary syndromes: ten year follow-up of the PRAIS-UK registry. Int J Cardiol 168:490–494
Michels KB, Yusuf S (1995) Does PTCA in acute myocardial infarction affect mortality and reinfarction rates? A quantitative overview (meta-analysis) of the randomized clinical trials. Circulation 91:476–485
Amsterdam EA, Wenger NK, Brindis RG et al (2014) HA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 130:e344–e426
Hamm CW, Bassand JP, Agewall S et al (2011) ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 32:2999–3054
Scirica BM, Bonaca MP, Braunwald E et al (2012) Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P-TIMI 50 trial. Lancet 380:1317–1324
Hoenig MR, Aroney CN, Scott IA (2010) Early invasive versus conservative strategies for unstable angina and non-ST elevation myocardial infarction in the stent era. Cochrane Database Syst Rev. doi:10.1002/14651858.CD004815.pub3
Smith SC Jr, Benjamin EJ, Bonow RO et al (2011) World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 124:2458–2473
Bhatt DL, Fox KA, Hacke W et al (2006) Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 354:1706–1717
Bhatt DL, Flather MD, Hacke W et al (2007) Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 49:1982–1988
Park SJ, Park DW, Kim YH et al (2010) Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med 362:1374–1382
Collet JP, Silvain J, Barthélémy O et al (2014) Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 384:1577–1585
Harjai KJ, Shenoy C, Orshaw P, Boura J (2009) Dual antiplatelet therapy for more than 12 months after percutaneous coronary intervention: insights from the Guthrie PCI Registry. Heart 95:1579–1586
Jacobs DR Jr, Kroenke C, Crow R et al (1999) PREDICT: a simple risk score for clinical severity and long-term prognosis after hospitalization for acute myocardial infarction or unstable angina: the Minnesota heart survey. Circulation 100:599–607
Fox KAA, Dabbous OH, Goldberg RJ et al (2006) Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 333:1091–1094
Roe MT, Chen AY, Thomas L et al (2011) Predicting long-term mortality in older patients after non-ST-segment elevation myocardial infarction: the CRUSADE long-term mortality model and risk score. Am Heart J 162(875–883):e1
Fox KAA, FitzGerald G, Puymirat E et al (2014) Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation, and outcomes using the updated GRACE risk score. BMJ Open 4:e004425
Pocock S, Bueno H, Licour M et al (2014) Predictors of one-year mortality at hospital discharge after acute coronary syndromes: a new risk score from the EPICOR (longtErm follow uP of antithrombotic management patterns In acute CORonary syndrome patients) study. Eur Heart J Acute Cardiovasc Care. doi:10.1177/2048872614554198
García-Alvarez A, Reguerio A, Hernández J et al (2014) Additional value of B-type natriuretic peptide on discrimination of patients at risk for mortality after a non-ST-segment elevation acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 3:132–140
Narayan H, Dhillon OS, Quinn PA et al (2011) C-terminal provasopressin (copeptin) as a prognostic marker after acute non-ST elevation myocardial infarction: leicester acute myocardial infarction peptide II (LAMP II) study. Clin Sci 121:79–89
Widera C, Pencina MJ, Meisner A et al (2012) Adjustment of the GRACE score by growth differentiation factor 15 enables a more accurate appreciation of risk in non-ST-elevation acute coronary syndrome. Eur Heart J 3:1095–1104
Chew DP, Junbo G, Parsonage W et al (2013) Perceived risk of ischemic and bleeding events in acute coronary syndromes. Circ Cardiovasc Qual Outcomes 6:299–308
Yan AT, Yan RT, Huynh T et al (2009) Understanding physicians’ risk stratification of acute coronary syndromes: insights from the Canadian ACS 2 Registry. Arch Intern Med 169:372–378
Antman EM, Cohen M, Bernink PJLM et al (2000) The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 284:835–842
Boersma E, Pieper KS, Steyerberg EW et al (2000) Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation 101:2557–2567
Lagerqvist B, Diderholm E, Lindahl B et al (2005) FRISC score for selection of patients for an early invasive treatment strategy in unstable coronary artery disease. Heart 91:1047–1052
Mahaffey KW, Yang Q, Pieper KS et al (2008) Prediction of one-year survival in high-risk patients with acute coronary syndromes: results from the SYNERGY trial. J Gen Intern Med 23:310–316
National Institute for Health and Clinical Excellence. Unstable angina and NSTEMI: the early management of unstable angina and non-ST-segment-elevation myocardial infarction. London: NICE (2010). https://www.nice.org.uk/guidance/cg94. Accessed 16 March 2015
Bueno H, Fernandez-Aviles F (2012) Use of risk scores in acute coronary syndromes. Heart 98:162–168
Fox KAA, Carruthers KF, Dunbar DR et al (2010) Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK–Belgian Study). Eur Heart J 31:2755–2764
Subherwal S, Bach RG, Chen AY et al (2009) Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) bleeding score. Circulation 119:1873–1882
Mathews R, Peterson ED, Chen AY et al (2011) In-hospital major bleeding during ST-elevation and non–ST-elevation myocardial infarction care: derivation and validation of a model the ACTION Registry®-GTWG™. Am J Cardiol 107:1136–1143
Lopes RD, Subherwal S, Holmes DN et al (2012) The association of in-hospital major bleeding with short-, intermediate-, and long-term mortality among older patients with non-ST-segment elevation myocardial infarction. Eur Heart J 33:2044–2053
Spencer FA, Moscucci M, Granger CB et al (2007) Does comorbidity account for the excess mortality in patients with major bleeding in acute myocardial infarction? Circulation 116:2793–2801
Collet JP, Montalescot G, Steg PG et al (2009) Clinical outcomes according to permanent discontinuation of clopidogrel or placebo in the CHARISMA trial. Arch Cardiovasc Dis 102:485–496
Mega JL, Braunwald E, Wiviott SD et al (2012) Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 366:9–19
Bottorff MB, Nutescu EA, Spinler S (2007) Antiplatelet therapy in patients with unstable angina and non-ST-segment-elevation myocardial infarction: findings from the CRUSADE National Quality Improvement Initiative. Pharmacotherapy 27:1145–1162
McNamara RL, Chung SC, Jernberg T et al (2014) International comparisons of the management of patients with non-ST segment elevation acute myocardial infarction in the United Kingdom, Sweden, and the United States: the MINAP/NICOR, SWEDEHEART/RIKS-HIA, and ACTION Registry-GWTG/NCDR registries. Int J Cardiol 175:240–247
Sherwood MW, Wiviott SD, Peng SA et al (2014) Early clopidogrel versus prasugrel use among contemporary STEMI and NSTEMI patients in the US: insights from the National Cardiovascular Data Registry. J Am Heart Assoc 3:e000849
Doucet J, Greboval-Furstenfeld E, Tavildari A et al (2008) Which parameters differ in very old patients with chronic atrial fibrillation treated by anticoagulant or aspirin? Antithrombotic treatment of atrial fibrillation in the elderly. Fundam Clin Pharmacol 22:569–574
Morrow DA, Braunwald E, Bonaca MP et al (2012) Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 366:1404–1413
Rapsomaniki E, Janzon M, Cohen DJ et al (2014) International comparison of outcomes among 140,887 survivors after acute myocardial infarction: real-world evidence from electronic health and administrative records. European Society of Cardiology Congress. Barcelona, 30 August–3 September 2014
Mauri L, Kereiakes DJ, Yeh RW et al (2014) Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 371:2155–2166
Yeh RW, Kereiakes DJ, Steg PG et al (2015) Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol. doi:10.1016/j.jacc.2015.03.003
Schüpke S, Mehilli J, Laugwitz K-L et al (2014) Randomized, double blind trial of 6 versus 12 months of dual antiplatelet therapy after DES implantation (ISAR-SAFE). Circulation 130:2106
Gilard M, Barragan P, Noryani AA et al (2015) 6- versus 24-month dual antiplatelet therapy after implantation of drug eluting stents in patients nonresistant to aspirin: the randomized, multicenter ITALIC trial. J Am Coll Cardiol 65:777–786
Bonaca MP, Bhatt DL, Braunwald E et al (2014) Design and rationale for the prevention of cardiovascular events in patients with prior heart attack using ticagrelor compared to placebo on a background of aspirin-thrombolysis in myocardial infarction 54 (PEGASUS-TIMI 54) trial. Am Heart J 167:437–444
Bonaca MP, Bhatt DL, Cohen M et al (2015) Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. doi:10.1056/NEJMoa1500857
Conflicts of interest
Marc Cohen is a member of the Speakers Bureau and also participates on Advisory boards for AstraZeneca, Merck, and Janssen Pharmaceuticals, and receives a significant amount of financial compensation for these activities. However, he received no compensation for the development of this review. The author acknowledges the contribution made by Dr Gautam Visveswaran (Newark Beth Israel Medical Center) in reviewing drafts of this article. Medical writing support (drafting, revising, and editing the review article) was provided by Catherine Lee (Gardiner-Caldwell Communications, Macclesfield, UK), and medical writing support for revising the paper in response to journal comments was provided by Jackie Phillipson (Zoetic Science [formerly Gardiner-Caldwell Communications], Macclesfield, UK); this assistance was funded by AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cohen, M. Long-term outcomes in high-risk patients with non-ST-segment elevation myocardial infarction. J Thromb Thrombolysis 41, 464–474 (2016). https://doi.org/10.1007/s11239-015-1227-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-015-1227-1