Abstract
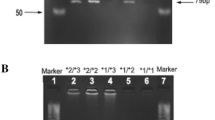
Warfarin is a widely used anticoagulant characterized by having a narrow therapeutic index and exhibiting a wide range of inter-individual and inter-ethnic variation. Single nucleotide polymorphisms in hepatic VKORC1 and CYP2C9 genes causes decreased and increased metabolism of warfarin respectively. The objective of this study was to evaluate the allele frequency of CYP2C9 polymorphic variants *2 and *3 and the association of these allelic variants with PT/INR and daily/weekly dose of warfarin. Seventy-four patients with heart valve replacement were selected. Patients taking low warfarin dose (4.90–17.50 mg weekly) for at least last 3 months and had a stable INR in the range of 2–3 were included in this study. CYP2C9 polymorphism was analyzed by polymerase chain reaction followed by restriction fragment length polymorphism (PCR–RFLP) technique. Among 74 patients, 9 (12.1 %) showed to have *2 allele, whereas 11 (14.1 %) had *3 allele. Genotype frequencies of wild and variant alleles were, 54.1, 17.6, 21.6 and 6.8 % for *1/*1, *1/*2, *1/*3 and *2/*3 respectively. None of the patient was homozygous for *2 and *3. Statistical analysis showed that low warfarin dose (weekly) is significantly associated with *1/*2 and *1/*3 genotypes (p value ≥ 0.001), whereas PT/INR showed no significant association with the any genotypes of CYP2C9. Our study suggest that polymorphic variants of CYP2C9 (*2 and *3) might influence warfarin dose requirements and associated with the low dose of warfarin in patients.


Similar content being viewed by others
References
Daniels CJ, Barbetseas J, Boudoulas H (2009) Prosthetic valves. In: American College of Cardiology (ed) Adult clinical cardiology self-assessment program, version 7, vol 4. American College of Cardiology, Washington, DC, pp 9.1–9.25
Vahanian A, Baumgartner H, Bax J et al (2007) Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European Society of Cardiology. Eur Heart J 28:230–268
Laplace G, Lafitte S, Labeque JN et al (2004) Clinical significance of early thrombosis after prosthetic mitral valve replacement. J Am Coll Cardiol 43:1283–1290
Pirmohamed M (2006) Warfarin: almost 60 years old and still causing problems. Br J Clin Pharmacol 62:509–511
Antonella T, Anne G (2004) Warfarin from rat poison to oral anticoagulant. Chronic ill 8:16–19
Nutescu EA, Shapiro NL, Chevalier A et al (2005) A pharmacologic overview of current and emerging anticoagulants. Clevel Clin J Med 72:S2–S6
Hirsh J, Fuster V, Ansell J, Halperin LJ (2003) American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation 107:1692–1711
Rieder MJ et al (2005) Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 352:2285–2293
Conce EA et al (2005) The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood 106:2329–2333
Lee CR, Goldstein JA, Pieper JA (2002) Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in vitro and human data. Pharmacogenetics 12:251–263
Takahashi H, Echizen H (2001) Pharmacogenetics of warfarin elimination and its clinical implications. Clin Pharmacokinet 40:587–603
Urquhart BL, Tirona RG, Kim RB (2007) Nuclear receptors and the regulation of drug metabolizing enzymes and drug transporters implications for inter-individual variability in response to drugs. J Clin Pharmacol 47:566–578
Sim SC, Ingelman-Sundberg M (2006) The human cytochrome P450 allele nomenclature committee web site: submission criteria, procedures, and objectives. In: Phillips IR, Shephard EA (eds) Cytochrome P450 protocols. Methods in molecular biology, vol 320. Humana Press, pp 183–191
Rettie AE, Wienkers LC, Gonzalez FJ, Trager WF, Korzekwa KR (1994) Impaired S-warfarin metabolism catalyzed by R144C allelic variant of CYP2C9. Pharmacogenetics 4:39–42
Xie HG, Prasad S, Kim HC, Stein CM (2002) CYP2C9 allelic variants: ethnic distribution and functional significance. Adv Drug Deliv Rev 54(10):1257–1270
Kirchheiner J, Störmer E, Meisel C, Steinbach N, Roots I, Brockmöller J (2003) Influence of CYP2C9 genetic polymorphisms on pharmacokinetics of celecoxib and its metabolites. Pharmacogenetics 13(8):473–480
Caldwell MD, Berg RL, Zhang KQ, Glurich I, Schmelzer JR, Yale SH, Vidaillet HJ, Burmester JK (2007) Evaluation of genetic factors for warfarin dose prediction. Clin Med Res 5:8–16
Millican EA, Lenzini PA, Milligan PE, Grosso L, Eby C, Deych E et al (2007) Genetic based dosing in orthopedic patients beginning warfarin therapy. Blood 110:1511–1515
García-Martín E, Martínez C, Ladero JM, Gamito FJ, Agúndez JA (2001) High frequency of mutations related to impaired CYP2C9 metabolism in a Caucasian population. Eur J Clin Pharmacol 57:47–49
Gaikovitch EA, Cascorbi I, Mrozikiewicz PM et al (2003) Polymorphisms of drug-metabolizing enzymes CYP2C9, CYP2C19, CYP2D6, CYP1A1, NAT2 and of P-glycoprotein in a Russian population. Eur J Clin Pharmacol 59:303–312
Yasar U, Aklillu E, Canaparo R et al (2002) Analysis of CYP2C9*5 in Caucasian, oriental and Black-African populations. Eur J Clin Pharmacol 58:555–558
Aynacioglu AS, Brockmöller J, Bauer S et al (1999) Frequency of cytochrome P450 CYP2C9 variants in a Turkish population and functional relevance for phenytoin. Br J Clin Pharmacol 48:409–415
Imai J, Ieiri I, Mamiya K et al (2000) Polymorphism of the cytochrome P450 (CYP) 2C9 gene in Japanese epileptic patients: genetics analysis of the CYP2C9 locus. Pharmacogenetics 10:85–89
Yoon YR, Shon JH, Kim MK et al (2001) Frequency of cytochrome P450 2C9 mutant alleles in a Korean population. Br J Clin Pharmacol 51:277–280
Adithan C, Gerard N, Vasu S et al (2003) Allele and genotype frequency of CYP2C9 in Tamilnadu population. Eur J Clin Pharmacol 59:707–709
Zand N, Tajik N, Moghaddam AS, Milanian I (2007) Genetic polymorphisms of cytochrome P450 enzymes 2C9 and 2C19 in a healthy Iranian population. Clin Exp Pharm Physiol 34:102–105
Sambrook J, Rusell D (2001) Molecular cloning: a laboratory manual, vol 3, 3rd edn
Lindh JD, Holm ML, Rane A (2009) Influence of CYP2C9 genotype on warfarin dose requirements—a systematic review and meta-analysis. Eur J Clin Pharmacol 65(4):365–375
Oner OG, Langaee TY, Feng H, Buyru N, Ulutin T, Hatemi AC et al (2008) VKORC1 and CYP2C9 polymorphisms are associated with warfarin dose requirements in Turkish patients. EurJ Clin Pharmacol 64(9):889–894
Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, Wood P (2005) The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood 106(7):2329–2333
Kimmel S, French B, Kasner S et al (2013) A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med 369(24):2283–2293
Bazan NS, Mokhtar S, Sabry NA, Badary OA, Rizk A (2014) Factors affecting warfarin dose requirements and quality of anticoagulation in adult Egyptian patients: role of gene polymorphism. Iran J Med Sci 183:161–172
Jose R et al (2005) CYP2C9 and CYP2C19 genetic polymorphisms: frequencies in the south Indian population. Fundam Clin Pharmacol 19(1):101–105
Wang SL, Huang JD, Lai MD, Tsai JJ (1995) Detection of CYP2C9 polymorphism based on the polymerase chain reaction in Chinese. Pharmacogenetics 5(1):37–42
Santos PC et al (2013) CYP2C9 and VKORC1 polymorphisms influence warfarin dose variability in patients on long-term anticoagulation. Eur J Clin Pharmacol 69(4):789–797
Chern HD et al (2006) CYP2C9 polymorphism and warfarin sensitivity in Taiwan Chinese. Clin Chim Acta 367(1–2):108–113
You JH, Tsui KK, Wong RS, Cheng G (2012) Cost-effectiveness of dabigatran versus genotype-guided management of warfarin therapy for stroke prevention in patients with atrial fibrillation. PLos One 7(6):e39640
Taube J, Halsall D, Baglin T (2000) Influence of cytochrome P-450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over-anticoagulation in patients on long-term treatment. Blood 96(5):1816–1819
Crespi CL, Miller VP (1997) The R144C change in the CYP2C9*2 allele alters interaction of the cytochrome P450 with NADPH: cytochrome P450 oxidoreductase. Pharmacogenetics 7:203–210
Aithal GP, Day CP, Kesteven P, Daly AK (1999) Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 353:717–719
Acknowledgments
Author is thankful to all department fellows for giving kind support and help. We are also thankful to Dr. Shah Jahan, Dr. Saba and Shabbir Hussain for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yasmeen, F., Ghafoor, M.B., Khalid, A.W. et al. Analysis of CYP2C9 polymorphisms (*2 and *3) in warfarin therapy patients in Pakistan. Association of CYP2C9 polymorphisms (*2 and*3) with warfarin dose, age, PT and INR. J Thromb Thrombolysis 40, 218–224 (2015). https://doi.org/10.1007/s11239-015-1215-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-015-1215-5