Abstract
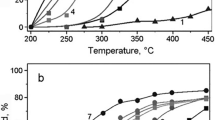
A study was carried out on the properties of Ni/Al2O3 and Cu-ZnO/Al2O3 composites supported on ceramic honeycomb monoliths made from synthetic cordierite in the carbon dioxide conversion of methane and the partial oxidation of methanol. The structured nickel-alumina catalysts are significantly more efficient than the conventional granulated catalysts. The improved working stability of these catalysts was achieved by adjusting the acid-base properties of the surface by introducing sodium and potassium oxides, which leads to inhibition of surface carbonization. The hydrogen yield was close to 90% in the partial oxidation of methanol with a stoichiometric reagent ratio in the presence of the Cu-ZnO/Al2O3/cordierite catalyst. A synergistic effect was found, reducing the selectivity of CO formation in the presence of the Cu-ZnO catalyst relative to samples derived from the individual components Cu and ZnO.
Similar content being viewed by others
References
Ch. Song and P. Wei, Catal. Today, 98, 463–484 (2004).
O. V. Krylov, Ros. Khim. Zh., 44, No. 1, 19–33 (2000).
S.-H. Lee, W. Cho, W.-S. Ju, et al., Catal. Today, 87, 133–137 (2003).
B.-Q. Xu, J.-M. Wei, Y.-T. Yu, et al., J. Phys. Chem. B, 107, 5203–5207 (2003).
O. Toshihiko and M. Toshiaki, J. Catal., 204, No. 1, 89–97 (2001).
E. I. Klabunovskii, V. P. Mordovin, and O. V. Krylov, Katal. Khim. Neftekhim. Prom., No. 6, 3–9 (2004).
K. Geissler, E. Newson, F. Vogel, et al., Phys. Chem. Chem. Phys., 3, 289–293 (2001).
L. A. Espinosa, R. M. Lago, M. A. Peña, and J. L. G. Fierro, Topics Catal., 22, Nos. 3/4, 245–251 (2003).
S. Schuyten and E. E. Wolf, Catal. Lett., 106, Nos. 1/2, 7–14 (2006).
H. Purnama, F. Girgsdies, T. Ressler, et al., Catal. Lett., 94, Nos. 1/2, 61–68 (2004).
Z. Hou, O. Yokota, T. Tanaka, and T. Yashima, Catal. Lett., 89, Nos. 1/2, 121–127 (2003).
V. Yu. Bychkov, O. V. Krylov, and V. N. Korchak, Kinet. Katal., 43, No. 1, 94–103 (2002).
V. Yu. Bychkov, Yu. P. Tyulenin, O. V. Krylov, and V. N. Korchak, Kinet. Katal., 43, No. 5, 775–782 (2002).
M. C. J. Bradford and M. A. Vannice, Appl. Catal. A, 142, No. 1, 73–96 (1996).
A. A. Firsova, Yu. P. Tyulenin, T. I. Khomenko, et al., Kinet. Katal., 44, No. 6, 893–901 (2003).
A. Yu. Kapran and S. N. Orlik, Teor. Éksp. Khim., 41, No. 6, 360–363 (2005). [Theor. Experim. Chem., 41, No. 6, 377–381 (2005) (Engl. Transl.).]
A. A. Ketov, D. V. Saulin, I. S. Puzanov, et al., Zh. Prikl. Khim., 70, No. 3, 446–450 (1997).
V. Yu. Bychkov, V. N. Korchak, O. V. Krylov, et al., Kinet. Katal., 42, No. 4, 618–631 (2001).
V. S. Artyunov and O. V. Krylov, Usp. Khim., 74, No. 12, 1216–1244 (2005).
J.-H. Kim, D. J. Suh, T.-J. Park, et al., V International Natural Gas Conversion Symposium, Giardini-Naxos, Sicily, 20–25 September, 1998, Elsevier, Amsterdam (1998), pp. 771–776.
Z. Xu, Y. Li, J. Zhang, and R. Zhou, Appl. Catal. A, 213, No. 1, 65–71 (2001).
T. Osaki and T. Mori, J. Catal., 204, No. 1, 89–97 (2001).
P. H. Matter, D. J. Braden, and U. S. Ozkan, J. Catal., 223, 340–351 (2004).
X.-R. Zhang, L.-C. Wang, C.-Z. Yao, et al., Catal. Lett., 10, Nos. 3/4, 183–190 (2005).
B. L. Kniep, F. Girgsdies, and T. Ressler, J. Catal., 236, 34–44 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 43, No. 5, pp. 299–306, September–October, 2007.
Rights and permissions
About this article
Cite this article
Solov’ev, S.A., Kapran, A.Y. & Orlik, S.N. Oxidative conversion of methane and methanol on M/Al2O3/cordierite structured metal oxide catalysts (m = Ni, Cu, Zn). Theor Exp Chem 43, 325–333 (2007). https://doi.org/10.1007/s11237-007-0040-0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11237-007-0040-0