Abstract
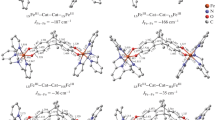
To find components of the magnetic sub-lattice of bi-functional compounds with maximum ferromagnetic exchange coupling, quantum-chemical calculations of complexes [(L)2M1III(L)M2II(L)2]5- are performed (M1III and M2II are tri- and divalent atoms of 3d and 4d transition metals M1III (Cr, Mo) and M2II (Ni, Co, Tc, Ru, Rh, Pd), L is dithiooxamide or oxalate). Calculations of the geometric structure of the complex are performed at the B3LYP/LANL2DZ level and calculations of J constants are performed at the B3LYP/TZV level. The replacement of a divalent atom of 3d metal by a divalent atom of 4d metal leads to an increase of J, whereas the replacement of a trivalent atom of 3d metal by a trivalent atom of 4d metal does not change the value of J. It is concluded that there is a correlation of J with a total change in the spin density on the M1III and M2II metals in the complexes compared to the M13+ and M22+ isolated cations.

Quantum-chemical modeling of exchange coupling in the magnetic su-blattice of bi-functional compounds containing heterometallic complexes of 3d and 4d metals with oxalate and dithiooxamide ligands.
Sergey M. Aldoshin, KonstantinV. Bozhenko, Andrey N. Utenyshev,
By means of DFT calculations of the complexes [(L)2M1III(L)M2II(L)2]5- it is found that there is a correlation of the exchange coupling constant J (red line) with the absolute value of total SD changes in the metals when moving from isolated cations M13+ and M22+ to complexes. This fact clarifies yet another aspect of the physical meaning of J.









Similar content being viewed by others
Notes
The structural parameters of the complexes and their symmetry are given in supplementary section.
References
Kahn O, Larionova J, Ouahab L (1999) Magnetic anisotropy in cyano-bridged bimetallic ferromagnets synthesized from the [Mo(CN)7]4- precursor. Chem Commun (Camb) 11:945–952. doi:10.1039/a900180h
Larionova J, Mombelli B, Sanchiz J, Kahn O (1998) Magnetic properties of the two-dimensional bimetallic compounds (NBu4)[MIIRuIII(ox)3] (NBu4 = Tetra-n-butylammonium; M = Mn, Fe, Cu; ox = oxalate). Inorg Chem 37:679–684. doi:10.1021/ic970531x
Larionova J, Kahn O, Bartolome J, et al. (1999) Heat capacity, alternating current magnetic susceptibilities, and pressure effect for the cyano-bridged bimetallic ferromagnet Mn2(H2O)5Mo(CN)7·4H2O (α phase). Chem Mater 11:3400–3405. doi:10.1021/cm991117t
Larionova J, Kahn O, Golhen S, et al. (1999) Magnetic transitions in the cyano-bridged bimetallic ferromagnet Mn2(H2O)5Mo(CN)7·4.75H2O (β phase). Inorg Chem 38:3621–3627. doi:10.1021/ic981262t
Larionova J, Kahn O, Gohlen S, et al. (1999) Structure, ferromagnetic ordering, anisotropy, and spin reorientation for the two-dimensional cyano-bridged bimetallic compound K2Mn3(H2O)6[Mo(CN)7]2·6H2O. J Am Chem Soc 121:3349–3356. doi:10.1021/ja9832717
Ruiz E, Rodriguez-Fortea A, Alvarez S, Verdaguer M (2005) Is it possible to get high TC magnets with Prussian blue analogues? A theoretical prospect. Chem Eur J 11:2135–2144. doi:10.1002/chem.200400821
Freedman DE, Jenkins DM, Long JR (2009) Strong magnetic exchange coupling in the cyano-bridged coordination clusters [(PY5Me2)4V4M(CN)6]5+ (M = Cr, Mo). Chemical Communications (Cambridge, United Kingdom) 32:4829–4831. doi:10.1039/b908736b
Shores MP, Sokol JJ, Long JR (2002) Nickel(II)-molybdenum(III)-cyanide clusters: synthesis and magnetic behavior of species incorporating [(Me3tacn)Mo(CN)3]. J Am Chem Soc 124:2279–2292. doi:10.1021/ja011645h
Rinehart JD, Harris TD, Kozimor SA, et al. (2009) Magnetic exchange coupling in actinide-containing molecules. Inorg Chem 48:3382–3395. doi:10.1021/ic801303w
Benelli C, Gatteschi D (2002) Magnetism of lanthanides in molecular materials with transition-metal ions and organic radicals. Chem Rev 102:2369–2387
Roman P, Guzman-Miralles C, Lugue A, Beitia JI, Cano J, Lloret F, Julve M, Alvarez S (1996) Influence of the peripheral ligand atoms on the exchange interaction in oxalato-bridged nickel (II) complexes: an orbital model. Crystal structures and magnetic properties of (H3dien) 2 [Ni2 (ox) 5] ⊙ 12H2O and [Ni2 (dien) 2 (H2O) 2 (ox)] Cl2. Inorg Chem 35:3741–3751
Rashid S, Turner S S, Day P, Light M E, and Hursthouse M B (2000) Molecular charge-transfer salt of BEDT–TTF [bis(ethylenedithio)tetrathiafulvalene] with the oxalate-bridged dimeric anion [Fe2(C2O4)5]4-. Inorg Chem 39: 2426–2428. doi: 10.1021/ic991409w
Armentano D, Munno G D, Faus J, Lloret F, and Julve M (2001) Syntheses, crystal structures, and magnetic properties of the oxalato-bridged mixed-valence complexes [FeII(bpm)3]2[FeIII 2(C2O4)5]·8H2O and FeII(bpm)3Na(H2O)2Fe(C2O4)3·4H2O (bpm = 2,2′-bipyrimidine). InorgChem 40:655–660.
Zhong Z -J, Matsumo N, Okawa H and Kida S (1990) 4[CuCr(ox)3]}x and {[{Cu(bpy)}2Cr(ox)3]NO3}x. Chem. Lett. 19: 87–90.
Shilov GV, Ovanesyan NS, Sanina NA, Atovmyan LO, Gruziel M (2001) The enantioselective role of the [N(C5H11)4]+ organic cation in the structure of the molecular magnet [N(C5H11)4][MnIIFeIII(C2O4)3]. Russ J Coord Chem, 27:567–573.
Bozhenko KV, Korchagin DV, Utenyshev AN, et al. (2010) Computer search for new bridging ligands in magnetic sublattices of bifunctional materials. Russ. J. Management systems and information Technology 39(292):294
Korchagin DV, Utenyshev AN, Bozhenko KV, et al. (2011) Magnetic exchange coupling in transition metal complexes with bidentate bridging ligands: a quantum chemical study. Russ Chem Bull 60:1040–1044
Desplanches C, Ruiz E, Alvarez S (2003) Exchange coupling in metal complexes of the second transition series: a theoretical exploration. Eur J Inorg Chem 9:1756–1760. doi:10.1002/ejic.200200457
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision D.01. Gaussian, Inc., Wallingford, CT.
Neese F (2012) The ORCA program system. Wiley interdisciplinary reviews—computational molecular. Science 2:73–78
Noodleman L (1981) Valence bond description of antiferromagnetic coupling in transition metal dimers. J Chem Phys 74:5737–5743. doi:10.1063/1.440939
Ginsberg AP (1980) Magnetic exchange in transition metal complexes. 12. Calculation of cluster exchange coupling constants with the Xα-scattered wave method. J Am Chem Soc 102:111–117. doi:10.1021/ja00521a020
Noodleman L, Davidson ER (1986) Ligand spin polarization and antiferromagnetic coupling in transition metal dimmers. Chem Phys 109:131–143. doi:10.1016/0301-0104(86)80192-6
Bencini A, Gatteschi D (1986) Xα-SW calculations of the electronic structure and magnetic properties of weakly coupled transition-metal clusters. The [Cu2Cl6]2- dimers. J Am Chem Soc 108:5763–5771. doi:10.1021/ja00279a017
Soda T, Kitagawa Y, Onishi T, et al. (2000) Ab initio computations of effective exchange integrals for H-H, H-He-H, and Mn2O2 complex: comparison of broken-symmetry approaches. Chem Phys Lett 319:223–230. doi:10.1016/S0009-2614(00)00166-4
Yamaguchi K, Takahara Y, Fueno T (1986) Ab-initio molecular orbital studies of structure and reactivity of transition metal-oxo compounds. Edited by Smith, Vedene H., Jr.; Schaefer, Henry F., III; Morokuma, Keiji, Appl. Quantum Chem., Proc. Nobel Laureate Symp 155–184. doi : 10.1007/978-94-009-4746-7_11
McKinnon SDJ, Gilroy JB, McDonald R, Patrick BO, Hicks RG (2011) Magnetostructural studies of palladium(II) and platinum(II) complexes of verdazyl radicals. J Mater Chem 21:1523–1530. doi:10.1039/c0jm02560g
Pointillart F, Train C, Villain F, et al. (2007) Six-fold oxygen-coordinated triplet (S = 1) palladium(II) moieties templated by tris(bipyridine)ruthenium(II) ions. J Am Chem Soc 129:1327–1334. doi:10.1021/ja066817v
Ruiz E, Rodriguez-Fortea A, Alvarez S, Verdaguer M (2005) Is it possible to get high TC magnets with Prussian blue analogues? A theoretical prospect. Chem Eur J 11:2135–2144. doi:10.1002/chem.200400821
Acknowledgements
The authors express their gratitude to M.Z. Aldoshina and T.L. Krivskaya.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 628 kb)
Rights and permissions
About this article
Cite this article
Aldoshin, S.M., Bozhenko, K.V. & Utenyshev, A.N. Quantum-chemical modeling of exchange coupling in the magnetic sublattice of bifunctional compounds containing heterometallic complexes of 3d and 4d metals with oxalate and dithiooxamide ligands. Struct Chem 28, 965–974 (2017). https://doi.org/10.1007/s11224-016-0900-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0900-0