Abstract
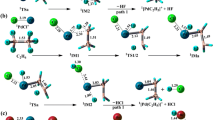
The reaction mechanism of the gas-phase PtCH2 + with H2S has been systematically investigated on the doublet and quartet potential energy surfaces at BPW91/6-311++G(2d, p)∪ SDD level. The Pt in PtCH2 + prefers to attack S–H bond in H2S. For PtCH2 + + H2S reaction, the potential energy surfaces (PESs), including three reaction pathways of hydrogen (including one and two hydrogen elimination) and methane elimination, have been explored and characterized. By contrast with hydrogen elimination, methane elimination reaction channel is energetically favorable, which is in good agreement with the experimental observation. The optimal S–H bond activation is the first step, followed by cleavage of Pt–C and Pt–S bond. About the path a and b, the lowering of activation barrier is mainly caused by the more stabilizing transition state interaction \(\varDelta E_{\text{int}}^{ \ne }\), which is the actual interaction energy between the deformed reactants in the transition state.





Similar content being viewed by others
References
Schwarz H (1991) Activation of methane. Angew Chem Int Ed Engl 30:820
Fox JM (1993) The different catalytic routes for methane valorization: an assessment of processes for liquid fuels. Catal Rev Sci Eng 35:169
Ashcroft AT, Cheetham AK, Foord JS, Green MLH, Grey CP, Murrell AJ (1990) Vemon PDF selective oxidation of methane to synthesis gas using transition metal catalysts. Nature 334:319
Lin M, Sen A (1994) Direct catalytic conversion of methane to acetic acid in an aqueous medium. Nature 368:613
Capitan MJ, Malet P, Centeno MA, Munoz-PAez A, Carrizos I, Odriozola JA (1993) Samarium oxide (Sm2O3)/alumina catalysts for methane coupling. Influence of the structure of surface samarium–aluminum–oxygen phases on the reactivity. J Phys Chem 97:9233
Irikura KK, Beauchamp JL (1991) Methane oligomerization in the gas phase by third-row transition-metal ions. J Am Chem Soc 113:2769
Irikura KK, Beauchamp JL (1991) Electronic structure considerations for methane activation by third-row transition-metal ions. J Phys Chem 95:8344
Irikura KK, Beauchamp JL (1989) Osmium tetroxide and its fragment ions in the gas phase: reactivity with hydrocarbons and small molecules. J Am Chem Soc 111:75
Achatz U, Berg C, Joos S, Fox BS, Beyer MK, Niedner-Schatteburg G (2000) Bondybey VE methane activation by platinum cluster ions in the gas phase: effects of cluster charge on the Pt4 tetramer. Chem Phys Lett 320:53
Koszinowski K, Schröder D, Schwarz H (2003) Reactivity of small cationic platinum clusters. J Phys Chem A 107:4999
Adlhart C, Uggerud E (2006) Reactions of platinum clusters Ptn±, n = 1 − 21, with CH4: to react or not to react. Chem Commun 24:2581
Heinemann C, Wesendrup R, Schwarz H (1995) Pt+-mediated activation of methane: theory and experiment. Chem Phys Lett 239:75
Pavlov M, Blomberg MRA, Siegbahn PEM, Wesendrup R, Heinemann C, Schwarz H (1997) Pt+-catalyzed oxidation of methane: theory and experiment. J Phys Chem A 101:1567
Cui Q, Musaev DG, Morokuma K (1998) Molecular orbital study of H2 and CH4 activation on small metal clusters. I. Pt, Pd, Pt2, and Pd2. J Chem Phys 108:8418
Xia F, Cao ZX (2006) Relativistic DFT studies of dehydrogenation of methane by Pt cationic clusters: cooperative effect of bimetallic clusters. J Phys Chem A 110:10078
Xiao L, Wang LC (2007) Methane activation on Pt and Pt4: a density functional theory study. J. Phys. Chem. B 111:1657
Heinemann C, Hertwig R, Wesendrup R, Koch W, Schwarz H (1995) Relativistic effects on bonding in cationic transition-metal-carbene complexes: a density-functional study. J Am Chem Soc 117:495
Rakowitz F, Marian CM, Schimmelpfennig B (2000) Ground and excited states of PtCH2 +: assessment of the no-pair Douglas–Kroll Ab initio model potential method. Phys Chem Chem Phys 2:24818
Brönstrup M, Schröder D, Schwarz H (1999) Platinum-mediated coupling of methane and small nucleophiles (H2O, PH3, H2S, CH3NH2) as a model for C–N, C–O, C–P, and C–S bond formation in the gas phase. Organometallics 18:1939
Werner H (1990) Komplexe von CO und seinen Verwandten: Eine Klasse metallorganischer Verbindungen feiert Geburtstag. Angew Chem 102:1109
Broadhurst PV (1985) Transition metal thiocarbonyl complexes. Polyhedron 4:1801
Diefenbach A, Bickelhaupt FM (2001) Oxidative addition of Pd to C–H, C–C and C–Cl bonds: importance of relativistic effects in DFT calculations. J Chem Phys 115:4030
Diefenbach A, de Jong GT, Bickelhaupt FM (2005) Activation of H–H, C–H, C–C and C–Cl bonds by Pd and PdCl−. understanding anion assistance in C–X bond activation. J Chem Theory Comput 1:286
de Jong GT, Bickelhaupt FM (2007) Catalytic carbon–halogen bond activation: trends in reactivity, selectivity, and solvation. J Chem Theory Comput 3:514
Ess DH, Houk KN (2008) Theory of 1, 3-dipolar cycloadditions: distortion/interaction and frontier molecular orbital models. J Am Chem Soc 130:10187
Bickelhaupt FM (1999) Understanding reactivity with Kohn–Sham molecular orbital theory: E2–S 2N mechanistic spectrum and other concepts. J Comput Chem 20:114
Jerzy M, Mark SGA (2008) Theoretical study of the reaction of Ti+ with propane. Theor Chem Acc 120:243
Armentrout PB (2007) Activation of C2H6 and C3H8 by gas-phase Mo+: potential energy surfaces and reaction mechanisms. Organometallics 26:5486
Santo ED, Santos M, Michelini MC, Marcalo J, Russo N, Gibson JK (2011) Gas-phase reactions of the bare Th2 + and U2 + ions with small alkanes, CH4, C2H6, and C3H8: experimental and theoretical study of elementary organoactinide chemistry. J Am Chem Soc 133:1955
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc., Wallingford CT
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098
Perdev JP, Chevary JA, Vosko SH, Jackson KA, Pederson MP, Singh DJ (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J Chem Phys 72:5639
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735
Carpenter JE, Weinhold F (1988) Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J Mol Struct (Theochem) 169:41
Zhang XG, Liyanage R, Armentrout PB (2001) Potential energy surface for activation of methane by Pt+: a combined guided ion beam and DFT study. J Am Chem Soc 123:5563
Pyykkö P, Riedel S, Patzschke M (2005) Triple-bond covalent radii. Chem Eur J 11:3511
Acknowledgments
This work was funded by the Scientific Study Project for Institutes of Higher Learning, Ministry of Education, Gansu Province (No. 2015A-140). The sixth group of college students’ science and technology innovation project (No. 113).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Tong, Y., Xu, X. et al. Theoretical study on the H2S activation by PtCH2 + in the gas phase. Struct Chem 27, 1363–1371 (2016). https://doi.org/10.1007/s11224-016-0756-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0756-3