Abstract
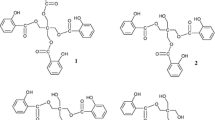
New estrane salicyloyloxy or D-homo derivatives were synthesized under microwave (MW) or conventional heating from estrane precursors and methyl salicylate. The MW technique provides advantages regarding product yield and reaction time, and represents a more environmentally friendly approach than conventional heating. Considering the biomedical potential of estrane compounds, we evaluated the antioxidant activity and cytotoxicity of synthesized estrane derivatives in a series of in vitro tests, as well as their 3β-hydroxysteroid dehydrogenase/Δ5 → Δ4 isomerase (3βHSD) and 17β-hydroxysteroid dehydrogenase types 1, 2 and 3 (17βHSD1, 17βHSD2 and 17βHSD3) inhibition potentials. In DPPH tests, 3-methoxyestra-1,3,5(10)-trien-17β-yl salicylate displayed antioxidant potential, while all compounds exhibited OH radical neutralization activity. 3-Oxoestr-4-en-17β-yl salicylate showed strong cytotoxicity against MDA-MB-231 breast cancer cells, while 17-oxoestra-1,3,5(10)-trien-3-yl salicylate, estra-1,3,5(10)-triene-3,17β-diyl 3-benzoate 17-salicylate and 3-benzyloxy-17-salicyloyloxy-16,17-secoestra-1,3,5(10)-triene-16-nitrile showed the strongest inhibition of PC-3 prostate cancer cell growth. 3-Hydroxyestra-1,3,5(10)-trien-17β-yl salicylate was the best inhibitor of 17βHSD2, suggesting potential use in treating pathological conditions associated with estrogen depletion. For 3-methoxyestra-1,3,5(10)-trien-17β-yl salicylate and 3-oxoestr-4-en-17β-yl salicylate, X-ray crystal structure analysis and molecular energy optimization were performed to define their conformations and energy minima. Very good overlap in the region of the steroidal nucleus was observed for the molecular structures of each analyzed molecule in the crystalline state and after energy optimization, while conformer analysis indicates conformational flexibility in the form of rotation around the C17···O2 bond. Structural geometry analysis for these compounds shows that the region of ring A in steroids, and especially the C3 atom functional group, is important structural features concerning antiproliferative activity against MDA-MB-231 cells.




Similar content being viewed by others
References
Rasmussen MK, Ekstrand B, Zamaratskaia G (2013) Int J Mol Sci 14:17926–17942
Vihko P, Herrala A, Härkönen P, Isomaa V, Kaija H, Kurkela R, Li Y, Patrikainen L, Pulkka A, Soronen P, Törn S (2005) J Steroid Biochem 93:277–283
Marchais-Oberwinkler S, Henn C, Müller G, Klein T, Negri M, Oster A, Spadaro A, Werth R, Wetzel M, Xu K, Frotscher M, Hartmann RW, Adamski J (2011) J Steroid Biochem Mol Biol 125:66–82
Tremblay MR, Lin SX, Poirier D (2001) Steroids 66:821–831
Maltais R, Ayan D, Trottier A, Barbeau X, Lagüe P, Bouchard JE, Poirier D (2014) J Med Chem 57:204–222
Maltais R, Trottier A, Delhommea A, Barbeau X, Lagüe P, Poirier D (2015) Eur J Med Chem 93:470–480
Szabó J, Bacsa I, Wölfling J, Schneider G, Zupkó I, Varga M, Herman BE, Kalmár L, Szécsi M, Mernyák E (2015) J Enzyme Inhib Med Chem. doi:10.3109/14756366.2015.1050008
Sam KM, Labrie F, Poirier D (2000) Eur J Med Chem 35:217–225
Bydal P, Auger S, Poirier D (2004) Steroids 69:325–342
Spires TE, Fink BE, Kick EK, You D, Rizzo CA, Takenaka I, Lawrence MR, Ruan Z, Salvati ME, Vite GD, Weinmann R, Attar RM, Gottardis MM, Lorenzi MV (2005) Prostate 65(2):159–170
Salvador JAR, Carvalho JFS, Neves MAC, Silvestre SM, Leitão AJ, Silva MMC, Sá e Melo ML (2013) Nat Prod Rep 30:324–374
Oklješa A, Jovanović-Šanta S, Klisurić O, Sakač M, Djurendić E, Jakimov D, Aleksić L, Penov Gaši K (2013) J Braz Chem Soc 24(10):1613–1622
Sakač MN, Gaković AR, Csanadi JJ, Djurendić EA, Klisurić O, Kojić V, Bogdanović G, Penov Gaši KM (2009) Tetrahedron Lett 50:4107–4109
Jovanović-Šanta SS, Andrić S, Andrić N, Bogdanović G, Petrović JA (2011) Med Chem Res 20:1102–1110
Penov Gaši KM, Oklješa AM, Petri ET, Ćelić AS, Djurendić EA, Klisurić OR, Csanadi JJ, Batta G, Nikolić AR, Jakimov DS, Sakač MN (2013) MedChemComm 4:317–323
Mernyák E, Fiser G, Szabó J, Bodnár B, Schneider G, Kovács I, Ocsovszki I, Zupkó I, Wölfling J (2014) Steroids 89:47–55
Mernyák E, Szabó J, Huber J, Schneider G, Minorics R, Bózsity N, Zupkó I, Varga M, Bikádi Z, Hazai E, Wölfling J (2014) Steroids 87:128–136
Römer W, Oettel M, Menzenbach B, Droescher P, Schwarz S (1997) Steroids 62:688–694
Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F (1997) Mol Pharmacol 51:535–541
Perez E, Cai ZY, Covey DF, Simpkins JW (2006) Drug Dev Res 66:78–92
Ott I, Schmidt K, Kircher B, Schumacher P, Wiglenda T, Gust R (2005) J Med Chem 48:622–629
Ekinci D, Sentürk M, Küfrevioglu OI (2011) Expert Opin Ther Pat 21(12):1831–1841
Fletcher S, Singh J, Zhang X, Yue P, Page BDG, Sharmeen S, Shahani VM, Zhao W, Schimmer AD, Turkson J, Gunning PT (2009) ChemBioChem 10(12):1959–1964
Djurendić EA, Dojčinović-Vujašković SV, Sakač MN, Jovin EDJ, Kojić VV, Bogdanović GM, Klisurić OR, Stanković SM, Lazar DV, Fabian L, Penov-Gaši KM (2010) Struct Chem 21:67–78
Djurendić E, Dojčinović-Vujašković S, Sakač M, Ajduković J, Gaković A, Kojić V, Bogdanović G, Klisurić O, Penov-Gaši K (2011) Arkivoc 2:83–102
Djurendić E, Savić M, Jovanović-Šanta S, Sakač M, Kojić V, Szécsi M, Oklješa A, Poša M, Penov Gaši K (2014) Acta Period Technol 45:173–189
Djurendić E, Klisurić O, Szécsi M, Sakač M, Jovanović-Šanta S, Ignáth I, Kojić V, Oklješa A, Savić M, Penov Gaši K (2014) Struct Chem 25:1747–1758
Penov Gaši KM, Djurendić EA, Szécsi M, Gardi J, Csanadi JJ, Klisurić OR, Dojčinović-Vujašković SV, Nikolić AR, Savić MP, Ajduković JJ, Oklješa AM, Kojić VV, Sakač MN, Jovanović-Šanta SS (2015) Steroids 94:31–40
Penov Gaši K, Djurendić E, Dojčinović Vujašković S, Gaković A, Jovanović-Šanta S, Kojić V, Sakač M (2012) Chem Pap 66(4):284–294
Klaunig JE, Kamendulis LM, Hocevar BA (2010) Toxicol Pathol 38:96–109
Butenandt A, Strömer I, Westphal V (1932) Z Physiol Chem 208:149–172
Jovanović-Šanta S, Petri E, Klisurić O, Szecsi M, Kovačević R, Petrović J (2015) Steroids 97:45–53
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren TJ, Bokesch H, Kenney S, Boyd RM (1990) J Natl Cancer Inst 82:1107–1112
Tóth I, Szécsi M, Julesz J, Faredin I (1997) Skin Pharmacol 10(3):160–168
Darvas B, Székács A, Fónagy A, Szécsi M, Tóth I (1997) Gen Comp Endocrinol 107(3):450–460
Oxford Diffraction CrysAlis CCD and CrysAlis RED (2008) Versions 1.171. Oxford Diffraction Ltd, Abingdon, Oxfordshire, England
Sheldrick GM (2008) Acta Crystallogr A 64:112–122
Altomare A, Cascarano G, Giacovazzo C, Guagliardi A (1993) J Appl Cryst 26:343–350
Bernardinelli G, Flack HD (1985) Acta Cryst A41:500–511
Nardelli MJ (1995) Appl Cryst 28:659–662
Spek AL (1998) PLATON, a multipurpose crystallographic tool. University of Utrecht, The Netherlands
Farrugia LJ (1999) J Appl Cryst 32:876–881
Jovanović-Šanta S, Andrić S, Kovačević R, Pejanović V (2000) Collect Czech Chem Commun 65:77–82
David-Beabes GL, Overman MJ, Petrofski JA, Campbell PA, de Marzo AM, Nelson WG (2000) Int J Oncol 17:1077–1086
Farrugia LJ (1997) J Appl Crystallogr 30:565
Cremer D, Pople JA (1975) J Am Chem Soc 97:1354–1358
Duax WL, Weeks CM, Rohrer DC (1976) In: Eliel EL, Allinger N (eds) Topics in stereochemistry, vol 2. Wiley, New York, pp 271–283
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4:17
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, van der Streek T (2006) J Appl Cryst 39:453–457
Clark RC, Reid JS (1995) Acta Cryst A51:887–897
Acknowledgments
Authors would like to thank the Hungary-Serbia IPA Cross-border Co-operation Programme (Project No. HUSRB/1002/214/133 RECODAC), Provincial Secretariat for Science and Technological Development of Vojvodina (Grant No. 114-451-946/2015-03) and Ministry of Education, Science and Technological Development of the Republic of Serbia for financial support (Grant No. 172021). The work of N. Szabó was supported by a PhD Fellowship of the Talentum Fund of Richter Gedeon Plc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klisurić, O.R., Szécsi, M., Djurendić, E.A. et al. Structural analysis and biomedical potential of novel salicyloyloxy estrane derivatives synthesized by microwave irradiation. Struct Chem 27, 947–960 (2016). https://doi.org/10.1007/s11224-015-0678-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0678-5