Abstract
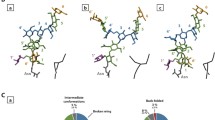
Glycoproteins play a central role in the immune response. In this study, we focus on the core of the common O-linked mucin-type glycopeptides. It has been observed that glycosylation stabilizes the protein in a stiffened, extended structure. We provide a unified picture for the conformation and stabilization of the O-glycosidic linkage using O-(2-acetylamino-2-deoxy-α- or -β-d-galacto- or -mannopyranosyl)-N-acetyl-l-serinamide model structures. We have calculated equilibrium geometries of the model structures with the B3LYP/6-31G(2df,p) method suggested in the Gaussian-4 theory. According to the relative energies, we confirm that the GalNAc-Ser linkage is more stable than its mannose analogues. The natural preference for the α-GalNAc-Ser over the β-GalNAc-Ser anomers can be explained by entropic effects. We explored the hydrogen bonding patterns on the carbohydrate unit calculating highly accurate dRPA@PBE0.25 and dRPA75 energies and found that in some cases, the acetamido group can be fixed by hydrogen bonding from the (O3Carb)H atom, but in most of the cases, it can rotate more freely. The torsion angles in the glycosidic linkage show that the linkage is stiffened more in the α-anomers and the most in the α-GalNAc-Ser structure because of the steric strains in the axial position and by two or three intramolecular hydrogen bonds. We also found that although, in our gas-phase model geometries, the peptide backbone prefers to be in a γ L-turn, a structural water molecule can stabilize a polyproline II helix of a proline-rich sequence, a β-sheet, or more likely random coils.







Similar content being viewed by others
References
Lis H, Sharon N (1993) Protein glycosylation. Structural and functional aspects. Eur J Biochem 218:1–27
Vliegenthart JF, Casset F (1998) Novel forms of protein glycosylation. Curr Opin Struct Biol 8:565–571
Khoury GA, Baliban RC, Floudas CA (2011) Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Sci Rep. doi:10.1038/srep00090
Caramelo JJ, Parodi AJ (2015) A sweet code for glycoprotein folding. FEBS Lett. doi:10.1016/j.febslet.2015.07.021
Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (2009) Essentials of glycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Gamblin DP, Scanlan EM, Davis BG (2009) Glycoprotein synthesis: an update. Chem Rev 109:131–163
Ambrosi M, Cameron NR, Davis BG (2005) Lectins: tools for the molecular understanding of the glycocode. Org Biomol Chem 3:1593–1608
Rudd PM, Dwek RA (1997) Glycosylation: heterogeneity and the 3D structure of proteins. Crit Rev Biochem Mol Biol 32:1–100
Kornfeld R, Kornfeld S (1976) Comparative aspects of glycoprotein structure. Annu Rev Biochem 45:217–237
Yasukata T (1999) Structure–activity relationship. Glycopeptide antibiotics. Nippon Yakuzaishikai Zasshi 51:215–221
Dwek RA (1996) Glycobiology: toward understanding the function of sugars. Chem Rev 96:683–720
Bertozzi CR, Kiessling LL (2001) Chemical glycobiology. Science 291:2357–2364
Cohen-Anisfeld ST, Lansbury PT (1993) A practical, convergent method for glycopeptide synthesis. J Am Chem Soc 115:10531–10537
Meldal M, Bock K (1994) A general approach to the synthesis of O- and N-linked glycopeptides. Glycoconj J 11:59–63
Sears P, Wong CH (1998) Enzyme action in glycoprotein synthesis. Cell Mol Life Sci 54:223–252
Seitz O (2000) Glycopeptide synthesis and the effects of glycosylation on protein structure and activity. Chem Bio Chem 1:214–246
Davis BG (2002) Synthesis of glycoproteins. Chem Rev 102:579–601. doi:10.1021/cr0004310
Spain SG, Gibson MI, Cameron NR (2007) Recent advances in the synthesis of well-defined glycopolymers. J Polym Sci A Polym Chem 45:2059–2072
Helenius A, Aebi M (2001) Intracellular functions of N-linked glycans. Science 291:2364–2369
Imperiali B, O’Connor SE (1999) Effect of N-linked glycosylatian on glycopeptide and glycoprotein structure. Curr Opin Chem Biol 3:643–649
Van den Steen P, Rudd PM, Dwek RA, Opdenakker G (1998) Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol 33:151–208
Hounsell EF, Davies MJ, Renouf DV (1996) O-linked protein glycosylation structure and function. Glycoconj J 13:19–26
Strous GJ, Dekker J (1992) Mucin-type glycoproteins. Crit Rev Biochem Mol Biol 27:57–92
Hang HC, Bertozzi CR (2005) The chemistry and biology of mucin-type O-linked glycosylation. Bioorganic Med Chem 13:5021–5034
Rudd PM, Elliott T, Cresswell P, Dwek RA (2001) Glycosylation and the immune system. Science 291:2370–2376
Danishefsky SJ, Allen JR (2000) From the laboratory to the clinic: a retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angew Chemie Int Ed 39:836–863
Davis BG (2004) Mimicking posttranslational modifications of proteins. Science 303:480–482
Wang L, Schultz PG (2005) Expanding the genetic code. Angew Chemie Int Ed 44:34–66
Dube DH, Prescher JA, Quang CN, Bertozzi CR (2006) Probing mucin-type O-linked glycosylation in living animals. Proc Natl Acad Sci USA 103:4819–4824
Nicolaou KC, Boddy CNC, Bräse S, Winssinger N (1999) Chemistry, biology, and medicine of the glycopeptide antibiotics. Angew Chemie Int Ed 38:2096–2152
Donadio S, Sosio M, Stegmann E, Weber T, Wohlleben W (2005) Comparative analysis and insights into the evolution of gene clusters for glycopeptide antibiotic biosynthesis. Mol Genet Genomics 274:40–50
Wolter F, Schoof S, Süssmuth R (2007) Synopsis of structural, biosynthetic, and chemical aspects of glycopeptide antibiotics. In: Wittmann V (ed) Glycopeptides and glycoproteins. Springer, Berlin, pp 143–185
James RC, Pierce JG, Okano A, Xie J, Boger DL (2012) Redesign of glycopeptide antibiotics: back to the future. ACS Chem Biol 7:797–804
Tsung-Lin L, Yu-Chen L, Syue-Yi L (2012) Combining biocatalysis and chemoselective chemistries for glycopeptide antibiotics modification. Curr Opin Chem Biol 16:170–178
Carlstedt I, Sheehan JK, Corfield AP, Gallagher JT (1985) Mucous glycoproteins: a gel of a problem. Essays Biochem 20:40–76
Shogren R, Gerken TA, Jentoft N (1989) Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: light-scattering studies of ovine submaxillary mucin. Biochemistry 28:5525–5536
Cyster JG, Shotton DM, Williams AF (1991) The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J 10:893–902
Gerken TA, Butenhof KJ, Shogren R (1989) Effects of glycosylation on the conformation and dynamics of O-linked glycoproteins: carbon-13 NMR studies of ovine submaxillary mucin. Biochemistry 28:5536–5543
Li F, Erickson HP, James JA, Moore KL, Cummings RD, McEver RP (1996) Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J Biol Chem 271:6342–6348
Maeji NJ, Inoue Y, Chujo R (1987) Conformational-determining role for the N-acetyl group in the O-glycosidic linkage, a-galNAc-Thr. Biopolymers 26:1753–1767
Maeji NJ, Inoue Y, Chuio R (1987) Conformational study of 0-glycosylated threonine containing peptide models. Int J Pept Protein Res 29:699–1987
Naganagowda GA, Gururaja TL, Satyanarayana J, Levine MJ (1999) NMR analysis of human salivary mucin (MUC7) derived O-linked model glycopeptides: comparison of structural features and carbohydrate–peptide interactions. J Pept Res 54:290–310
Live DH, Williams LJ, Kuduk SD, Schwarz JB, Glunz PW, Chen XT, Sames D, Kumar RA, Danishefsky SJ (1999) Probing cell-surface architecture through synthesis: an NMR-determined structural motif for tumor-associated mucins. Proc Natl Acad Sci USA 96:3489–3493
Coltart DM, Royyuru AK, Williams LJ, Glunz PW, Sames D, Koduk SD, Schwarz JB, Chen XT, Danishefsky SJ, Live DH (2002) Principles of mucin architecture: structural studies on synthetic glycopeptides bearing clustered mono-, di-, tri-, and hexasaccharide glycodomains. J Am Chem Soc 124:9833–9844
Csonka GI, Schubert G, Perczel A, Sosa CP, Csizmadia IG (2002) Ab initio conformational space study of model compounds of O-glycosides of serine diamide. Chem Eur J 8:4718–4733
Corzana F, Busto JH, Jiménez-Osés G, Asensio JL, Jiménez-Barbero J, Peregrina JM, Avenoza A (2006) New insights into α-GalNAc-Ser motif: influence of hydrogen bonding versus solvent interactions on the preferred conformation. J Am Chem Soc 128:14640–14648
Corzana F, Busto JH, Jiménez-Osés G, De Luis MG, Asensio JL, Jiménez-Barbero J, Peregrina JM, Avenoza A (2007) Serine versus threonine glycosylation: the methyl group causes a drastic alteration on the carbohydrate orientation and on the surrounding water shell. J Am Chem Soc 129:9458–9467
Corzana F, Busto JH, Engelsen SB, Jiménez-Barbero J, Asensio JL, Peregrina JM, Avenoza A (2006) Effect of beta-O-glucosylation on L-Ser and L-Thr diamides: a bias toward alpha-helical conformations. Chem Eur J 12:7864–7871
Fernández-Tejada A, Corzana F, Busto JH, Jiménez-Osés G, Jiménez-Barbero J, Avenoza A, Peregrina JM (2009) Insights into the geometrical features underlying β-O-GIcNAc glycosylation: water pockets drastically modulate the interactions between the carbohydrate and the peptide backbone. Chem Eur J 15:7297–7301
Pratt MR, Bertozzi CR (2005) Synthetic glycopeptides and glycoproteins as tools for biology. Chem Soc Rev 34:58–68
Cocinero EJ, Stanca-Kaposta EC, Dethlefsen M, Liu B, Gamblin DP, Davis BG, Simons JP (2009) Hydration of sugars in the gas phase: regioselectivity and conformational choice in N-acetyl glucosamine and glucose. Chem Eur J 15:13427–13434
Mallajosyula SS, MacKerell AD Jr (2011) Influence of solvent and intramolecular hydrogen bonding on the conformational properties of O-linked glycopeptides. J Phys Chem B 115:11215–11229
Fernández-Tejada A, Corzana F, Busto JH, Jiménez-Osés G, Peregrina JM, Avenoza A (2008) Non-natural amino acids as modulating agents of the conformational space of model glycopeptides. Chem Eur J 14:7042–7058
Corzana F, Busto JH, Marcelo F, Garcíadeluis M, Asensio JL, Martín-Santamaría S, Jiménez-Barbero J, Avenoza A, Peregrina JM (2011) Engineering O-glycosylation points in non-extended peptides: implications for the molecular recognition of short tumor-associated glycopeptides. Chem Eur J 17:3105–3110
Curtiss LA, Redfern PC, Raghavachari K (2007) Gaussian-4 theory. J Chem Phys 126:084108
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158
Heßelmann A (2012) Random-phase-approximation correlation method including exchange interactions. Phys Rev A 85:012517
Kállay M (2014) A systematic way for the cost reduction of density fitting methods. J Chem Phys 141:244113
Mezei PD, Csonka GI, Ruzsinszky A, Kállay M (2015) Construction and application of a new dual-hybrid random phase approximation. J Chem Theory Comput, submitted
Mezei PD, Csonka GI, Kállay M (2015) Accurate Diels-Alder reaction energies from efficient density functional calculations. J Chem Theory Comput 11:2879–2888
Acknowledgments
This project is supported by the Grants TÁMOP-4.2.1/B-09/1/KMR-2010-0002 and TÁMOP-4.2.2.B-10/1–2010-0009. The authors thank the Hungarian National Higher Education and Research Network for the computer time.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Magdolna Hargittai on the occasion of her 70th birthday.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mezei, P.D., Csonka, G.I. Unified picture for the conformation and stabilization of the O-glycosidic linkage in glycopeptide model structures. Struct Chem 26, 1367–1376 (2015). https://doi.org/10.1007/s11224-015-0666-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0666-9