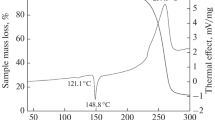
Abstract
5-Chloro-1,2,3,4-thiatriazole has been investigated in the gas phase for the first time by mid-infrared and He I photoelectron spectroscopy. The ground-state geometry has been obtained from quantum chemical calculations at the CCSD(T) and B3LYP levels using aug-cc-pVTZ basis set. Ionization potentials have been determined and the electronic structure has been discussed within the frame of molecular orbital theory. IR and photoelectron spectroscopies, supported by quantum chemical calculations at the B3LYP and SAC-CI levels, provide a detailed investigation into the vibrational and electronic character of the molecule. Thermal stability of 5-chloro-1,2,3,4-thiatriazole has been studied both experimentally and theoretically. Flash vacuum thermolysis of the molecule produces fast quantitatively N2, ClCN, and sulfur. Theoretical calculations at the CCSD(T)//B3LYP level predict competitive decomposition routes, starting either with a retro-cycloaddition reaction leading to N2S and ClCN or with a ring opening to chlorothiocarbonyl azide intermediate, to produce finally N2, S, and ClCN. Calculations also predict that N2S is reactive and decomposes in bimolecular reactions to N2 and S2.






Similar content being viewed by others
References
Freund M, Schander A (1896) Ueber das Amidotriazsulfol. Ber 29:2500–2505
Lieber E, Oftedahl E, Pillai CN, Hites RD (1957) Infrared Spectrum of the So-called 5-Amino-1,2,3,4-thiatriazole. J Org Chem 22:441–442
Bergtrup M (2003) 1,2,3,4-Thiatriazoles. Sci Synth 13:833–847
Jensen KA, Pedersen C (1964) 1,2,3,4-Thiatriazoles. Adv Heterocycl Chem 3:263–284
Holm A (1976) 1,2,3,4-Thiatriazoles. Adv Heterocycl Chem 20:145–174
Holm A, Larsen BD (1996) 1,2,3,4-Thiatriazoles. Comp Heterocycl Chem II 4:691–731
Dehaen W, Bakulev VA (2008) 1,2,3,4-Thiatriazoles. Comp Heterocycl Chem III 6:441–483
Lieber E, Lawyer CB, Trivedi JP (1961) Reaction of thiophosgene with azide ion. J Org Chem 26:1644–1646
Csákvári B, Nagy A, Zanathy L, Szepes L (1992) VUV photoelectron spectrometer (ATOMKI ESA 32) for multipurpose chemical applications. Magy Kém Foly 98:415–419
Frisch MJ et al (2010) GAUSSIAN 09 (Revision B.01), Gaussian, Inc., Wallingford CT
Pongor G (1993) Program scale 3, department of theoretical chemistry. Eötvös Loránd University, Budapest
Keresztury G, Jalsovszky G (1971) Alternative calculation of the vibrational potential energy distribution. J Mol Struct 10:304–305
Gordy W (1947) Dependence of bond order and of bond energy upon bond lengths. J Chem Phys 15:305–310
Bender H, Carnovale F, Peel JB, Wentrup C (1988) Dinitrogen sulfide, N2S, revealed by photoelectron spectroscopy. J Am Chem Soc 110:3458–3461
Wentrup C, Kambouris P (1991) N-Sulfides. dinitrogen sulfide, thiofulminic acid, and nitrile sulfides. Chem Rev 91:363–373
Krebsz M, Pasinszki T (2011) Generation, identification, and synthetic applications of nitrile sulfides and nitrile selenides. Curr Org Chem 15:1734–1744
Pasinszki T, Kárpáti T, Westwood NPC (2001) Structure and stability of small nitrile sulfides and their attempted generation from 1,2,5-thiadiazoles. J Phys Chem A 105:6258–6265
Seth-Paul WA (1969) Classical and modern procedures for calculating pr separations of symmetrical and asymmetrical top molecules. J Mol Struct 3:403–417
Pacsai B, Vass G, Pasinszki T (2012) Structure and spectroscopy of 3-chloro-4-fluoro-1,2,5-thiadiazole. Eur Chem Bull 1:98–102
Pasinszki T, Krebsz M, Vass G (2010) Ground and ionic states of 1,2,5-thiadiazoles: an UV-photoelectron spectroscopic and theoretical study. J Mol Struct 966:85–91
Frost DC, MacDonald CB, McDowell CA, Westwood NPC (1981) Preparation and He I photoelectron spectra of the halogen thiocyanates, XSCN (X = Cl and Br). J Am Chem Soc 103:4423–4427
Acknowledgments
We thank the Hungarian Scientific Research Fund for financial support to the project (grant no. OTKA K101164).
Author information
Authors and Affiliations
Corresponding author
Additional information
The paper is dedicated to Magdolna Hargittai on the occasion of her 70th birthday.
Rights and permissions
About this article
Cite this article
Pasinszki, T., Dzsotján, D., Vass, G. et al. Structure, spectroscopy, and thermal decomposition of 5-chloro-1,2,3,4-thiatriazole: a He I photoelectron, infrared, and quantum chemical study. Struct Chem 26, 1603–1610 (2015). https://doi.org/10.1007/s11224-015-0655-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0655-z