Abstract
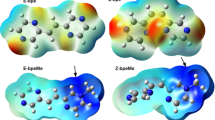
Cyclobutanetetrone, C4O4, has a triplet ground state, although the ground state of C4S4 is singlet. This computational study focuses on the mono-, di-, and trithiosquarate, C4O4−n S n (n = 1–3), molecules as transition stages between the two ending points (C4O4 and C4S4), and investigates the trends for the changes in the energies, geometry, partial atomic charges, partial spin densities, as well as orbital energies of the four low-lying electronic states. As the number of the sulfur atoms is increasing, the singlet spin state becomes energetically more and more preferred. For C4O3S molecule, where only one oxygen atom is substituted by sulfur, the CCSD(T) calculations predict a triplet ground state, but the error of the calculations is most likely higher than the calculated 0.5 kcal/mol singlet–triplet energy gap.





Similar content being viewed by others
Notes
We note that the discussed trends based on the enthalpy values at 0 K can be applied for the trends based on electronic energies. The zero point energy corrections usually favor to the actual ground electronic state, but the caused energy differences do not exceed the 0.7 kcal/mol. For the first two electronic states of C4O3S structure, where even very small corrections count, the electronic energy difference of the ground state triplet and the first exited state π 8 singlet is 0.5 kcal/mol (the ΔH 0 value is 0.6 kcal/mol).
References
Shiomi D, Tamura M, Sawa H, Kato R, Kinoshita M (1993) J Phys Soc Jpn 62:289–300
Tomioka H (2003) Pure Appl Chem 75:1041–1047
Huai P, Shimoi Y, Abe S (2005) Synth Met 152:469–472
Tanaka H, Ise T, Shiomi D, Sato K, Takui T (2006) J Low Temp Phys 142:605–608
Numata Y, Inoue K, Baranov N, Kurmoo M, Kikuchi K (2007) J Am Chem Soc 129:9902–9909
Ciofini I, Lainé PP, Zamboni M, Daul CA, Marvaud V, Adamo C (2007) Chem Eur J 13:5360–5377
Hayakawa K, Ise T, Shiomi D, Sato K, Takui T (2006) J Low Temp Phys 142:589–592
Iwamura H (2013) Polyhedron 66:3–14
Abe M (2013) Chem Rev 113:7011–7088
Gleiter R, Hyla-Kryspin I, Pfeifer KH (1995) J Org Chem 60:5878–5883
Jiao H, Frapper G, Halet JF, Saillard JY (2001) J Phys Chem A 105:5945–5947
Zhou X, Hrovat DA, Gleiter R, Borden WT (2009) Mol Phys 107:863–870
Zhou X, Hrovat DA, Borden WT (2010) J Phys Chem A 114:1304–1308
Guo JC, Hou GL, Li SD, Wang XB (2012) J Phys Chem Lett 3:304–308
Bao X, Zhou X, Lovitt CF, Venkatraman A, Hrovat DA, Gleiter R, Hoffmann R, Borden WT (2012) J Am Chem Soc 134:10259–10270
Bao X, Hrovat DA, Borden WT, Wang XB (2013) J Am Chem Soc 135:4291–4298
Bao X, Hrovat DA, Borden WT (2013) Chem Eur J 19:5687–5693
Zhang J, Hrovat DA, Sun Z, Bao X, Borden WT, Wang XB (2013) J Phys Chem A 117:7841–7846
Kozuch S, Hrovat DA, Borden WT (2013) J Am Chem Soc 135:19282–19291
Varga Z, Truhlar DG (2015) (to be published)
Stanton JF (1994) J Chem Phys 101:371–374
Yuan H, Cremer D (2000) Chem Phys Lett 324:389–402
Gräfenstein J, Kraka E, Filatov M, Cremer D (2002) Int J Mol Sci 3:360–394
Jacob CR, Reiher M (2012) Int J Quantum Chem 112:3661–3684
Yamaguchi K, Yoshioka Y, Fueno T (1977) Chem Phys Lett 46:360–365
Yamaguchi K, Yoshioka Y, Takatsuka K, Fueno T (1978) Theor Chim Acta 48:185–206
Yamaguchi K, Takahara Y, Fueno T, Houk KN (1988) Theor Chim Acta 73:337–364
Yamanaka S, Kawakami T, Nagao H, Yamaguchi K (1994) Chem Phys Lett 231:25–33
Kitagawa Y, Saito T, Ito M, Shoji M, Koizumi K, Yamanaka S, Kawakami T, Okumura M, Yamaguchi K (2007) Chem Phys Lett 442:445–450
Kitagawa Y, Saito T, Ito M, Nakanishi Y, Shoji M, Koizumi K, Yamanaka S, Kawakami T, Okumura M, Yamaguchi K (2007) Int J Quantum Chem 107:3094–3102
Saito T, Kitagawa Y, Shoji M, Nakanishi Y, Ito M, Kawakami T, Okumura M, Yamaguchi K (2008) Chem Phys Lett 456:76–79
Saito T, Nishihara S, Kataoka Y, Nakanishi Y, Matsui T, Kitagawa Y, Kawakami T, Okumura M, Yamaguchi K (2009) Chem Phys Lett 483:168–171
Kitagawa Y, Saito T, Nakanishi Y, Kataoka Y, Shoji M, Koizumi K, Kawakami T, Okumura M, Yamaguchi K (2009) Int J Quantum Chem 109:3641–3648
Saito T, Kataoka Y, Nakanishi Y, Matsui T, Kitagawa Y, Kawakami T, Okumura M, Yamaguchi K (2010) Chem Phys 368:1–6
Kitagawa Y, Saito T, Nakanishi Y, Kataoka Y, Matsui T, Kawakami T, Okumura M, Yamaguchis K (2010) Int J Quantum Chem 110:3053–3060
Coucouvanis D, Hollander FJ, West R, Eggerding D (1974) J Am Chem Soc 96:3006–3008
Seitz G, Mann K, Schmiedel R, Matusch R (1975) Chem-Ztg 99:90–91
Eggerding D, West R (1976) J Org Chem 41:3904–3909
Seitz G, Mann K, Schmiedel R, Matusch R (1979) Chem Ber 112:990–999
Zhao Y, Truhlar DG (2006) Theor Chem Acc 120:215–241
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision C.1; Gaussian, Inc., Wallingford, CT
Hirshfeld FL (1977) Theor Chim Acta 44:129–138
Marenich AV, Jerome SV, Cramer CJ, Truhlar DG (2012) J Chem Theory Comput 8:527–541
Wiberg K (1968) Tetrahedron 24:1083–1096
Lee TJ, Rice HE, Scuseria GE, Schaefer HF III (1989) Theor Chim Acta 75:81–98
Lee TJ, Taylor PR (1989) Int J Quantum Chem 23:199–207
Werner H-J, Knowles PJ, Knizia G, Manby FR, Schütz M (2012) WIREs Comput Mol Sci 2:242–253
Werner H-J, Knowles PJ, Knizia G, Manby FR, Schütz M, Celani P, Korona T, Lindh R, Mitrushenkov A, Rauhut G, Shamasundar KR, Adler TB, Amos RD, Bernhardsson A, Berning A, Cooper DL, Deegan MJO, Dobbyn AJ, Eckert F, Goll E, Hampel C, Hesselmann A, Hetzer G, Hrenar T, Jansen G, Köppl C, Liu Y, Lloyd AW, Mata RA, May AJ, McNicholas SJ, Meyer W, Mura ME, Nicklass A, O’Neill DP, Palmieri P, Peng D, Pflüger K, Pitzer R, Reiher M, Shiozaki T, Stoll H, Stone AJ, Tarroni R, Thorsteinsson T, Wang M (2012) MOLPRO, version 2012.1, a package of ab initio programs, see http://www.molpro.net
Knowles, PJ, Hampel C, Werner H-J (1993) J Chem Phys 99:5219-5227 (Erratum: (2000) J Chem Phys 112:3106–3107)
Alecu IM, Zheng J, Zhao Y, Truhlar DG (2010) J Chem Theory Comput 6:2872–2887
Papajak E, Leverentz HR, Zheng J, Truhlar DG (2009) J Chem Theory Comput 5:1197–1202
Lu T, Chen F (2012) J Comp Chem 33:580–592, see https://multiwfn.codeplex.com
Mayer I (1983) Chem Phys Lett 97:270–274
Mayer I, Salvador P (2004) Chem Phys Lett 383:368–375
Acknowledgments
This work was supported by computing time grants from Minnesota Supercomputing Institute. DGT acknowledges support from the U. S. Department of Energy, Office of Basic Energy Sciences, under Grant No. DE-SC0008666.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to Professor Magdolna Hargittai on the occasion of her 70th birthday.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Varga, Z., Truhlar, D.G. Singlet–triplet competition in the low-lying energy states of C4O4−n S n (n = 1–3) molecules. Struct Chem 26, 1229–1240 (2015). https://doi.org/10.1007/s11224-015-0633-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0633-5