Abstract
Kaempferol, one of the most bioactive plant flavonoids was experimentally and theoretically (at M05-2X/6-311G(d,p) level of theory) investigated for its ability to scavenge potentially, highly damaging hydroxyl and superoxide anion radicals. Relating the obtained hydroxyl radical activity sequence with kaempferol structural features, it could be assumed that C4′-OH functional most probably renders it as hydroxyl radical scavenger, while C5-OH group has more prominent role compared to ortho-hydroxy groups in B ring. However, kaempferol’s activity toward superoxide anion radical implicates ortho-hydroxy groups in B ring as more relevant. Theoretical calculations point to HAT and SPLET mechanisms as operative for kaempferol in all solvents under investigations.
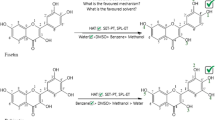
Graphical Abstract
The present paper aims to provide quantitative tools to thoroughly and comprehensively determine antiradical mechanisms of kaempferol in different media.




Similar content being viewed by others
References
Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel BT, Pieters L, Vlietinck AJ, Vanden Bergh D (1998) Structure-Activity Relationship and Classification of Flavonoids as Inhibitors of Xanthine Oxidase and Superoxide Scavengers. J Nat Prod 61:71–76
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Rice-Evans C (2001) Flavonoid antioxidants. Curr Med Chem 8:797–807
Wright JS, Johnson ER, DiLabio GA (2001) Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123(6):1173–1183
Mayer JM (2004) Proton-coupled electron transfer: a Reaction Chemist’s View. Annu Rev Phys Chem 55:363–390
DiLabio GA, Ingold KU (2005) A theoretical study of the iminoxyl/oxime self-exchange reaction. a five-center, cyclic proton-coupled electron transfer. J Am Chem Soc 127:6693–6699
DiLabio GA, Johnson ER (2007) Lone Pair-π and π -π interactions play an important role in proton-coupled electron transfer reactions. J Am Chem Soc 129:6199–6203
Sjodin M, Styring S, Åkermark B, Sun L, Hammarstrom L (2000) Proton-coupled electron transfer from tyrosine in a tyrosine − ruthenium − tris-bipyridine complex: comparison with tyrosinez oxidation in photosystem II. J Am Chem Soc 122:3932–3936
Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M (2011) Review on the dietary flavonoid kaempferol. Mini Rev Med Chem 11:298–344
Lopes GKB, Schulman HM, Hermes-Lima M (1999) Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta 1472:142–152
Jackson SK, Liu KJ, Liu M, Timmins GS (2002) Detection and removal of contaminating hydroxylamines from the spin trap DEPMPO, and re-evaluation of its use to indicate nitrone radical cation formation and S(N)1 reactions. Free Radic Biol Med 32(3):228–232
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Shakila G, Periandy S, Ramalingam S (2011) Molecular Structure and Vibrational Analysis of 1-Bromo-2-Chlorobenzene Using ab initio HF and Density Functional Theory (B3LYP) Calculations. J Atom Mol Optical Phys 1-10
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JAJr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega NJ, Millam M, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1-SMP, Gaussian Inc Wallingford CT
Marković Z, Milenković D, Đorović J, Dimitrić Marković JM, Stepanić V, Lučić B, Amić D (2012) Free radical scavenging activity of morin 2′-O- phenoxide anion. Food Chem 135:2070–2077
Black G, Simmie JM (2010) Barrier heights for H-atom abstraction by HO2 from n-butanol-a simple yet exacting test for model chemistries. J Comput Chem 31:1236–1248
Galano A, Alvarez-Idaboy JR (2009) Guanosine + OH radical reaction in aqueous solution: a reinterpretation of the UV-Vis data based on thermodynamic and kinetic calculations. Org Lett 11:5114–5117
Galano A, Macias-Ruvalcaba NA, Medina-Campos ON, Pedraza-Chaverri J (2010) Mechanism of the OH radical scavenging activity of nordihydroguaiaretic acid: a combined theoretical and experimental study. J Phys Chem B 114:6625–6635
Marković ZS, Dimitrić Marković JM, Doličanin ĆB (2010) Mechanistic pathways for the reaction of quercetin with hydroperoxy radical. Theor Chem Acc 127:69–80
Zavala-Oseguera C, Alvarez-Idaboy JR, Merino G, Galano A (2009) OH radical gas phase reactions with aliphatic ethers: a variation transition state theory study. J Phys Chem A 113:13913–13920
Alberto ME, Russo N, Grand A, Galano A (2013) A phusicochemical examination of the free radical scavenging activity of Trolox: mechanism, kinetics and influence of the environment. Phys Chem Chem Phys 15:4642–4650
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Lü JM, Lin PH, Yao Q, Chen C (2010) Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med 14:840–860
Fang YZ, Yang S, Wu G (2002) Free radicals, antioxidants and nutrition. Nutrition 18:872–879
Dimitrić Marković JM, Marković ZS, Brdarić TP, Pavelkić VM, Jadranin MB (2011) Iron complexes of dietary flavonoids: combined spectroscopic and mechanistic study of their free radical scavenging activity. Food Chem 129:1567–1577
Dimitrić Marković JM, Marković ZS, Pašti IA, Brdarić TP, Popović-Bijelića A, Mojović M (2012) A joint application of spectroscopic, electrochemical and theoretical approaches in evaluation of the radical scavenging activity of 3-OH flavones and their iron complexes towards different radical species. Dalton Trans 41:7295–7303
Dimitrić Marković JM, Marković ZS, Brdarić TP, Filipović ND (2011) Comparative spectroscopic and mechanistic study of chelation properties of fisetin with iron in aqueous buffered solutions. Implications on in vitro antioxidant activity. Dalton Trans 40:457–4560
Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ (2006) Distinctive antioxidant and antiinflammatory effects of flavonols. J Agric Food Chem 54:9798–9804
Heijnen CG, Haenen GR, van Acker FA, van der Vijgh WJ, Bast A (2001) Flavonoids as peroxynitrite scavengers: the role of the hydroxyl groups. Toxicol In Vitro 15:3–6
Marković ZS, Mentus SV, Dimitrić Marković JM (2009) Electrochemical and density functional theory study on the reactivity of fisetin and its radicals: implications on in vitro antioxidant activity. J Phys Chem A 113:4170–4179
Hendrickson HP, Kaufman AD, Lunte C (1994) Electrochemistry of catechol-containing flavonoids. J Pharm Biomed Anal 12:325–334
Yang B, Arai K, Kusu F (2001) Electrochemical behaviors of quercetin and kaempferol in neutral buffer solution. Anal Sci 17:987–989
Rene A, Abasq ML, Hauchard D, Hapiot P (2010) How do phenolic compounds react toward superoxide ion? A simple electrochemical method for evaluating antioxidant capacity. Anal Chem 82:8703–8710
Rong ZY, Wang ZW, Zhao B (2013) A DFT study on the structural and antioxidant properties of three flavonols. Food Biophys 8:90–94
Acknowledgments
The authors acknowledge financial support of the Ministry of Science of the Republic of Serbia, Grants No. 172015 and 41005.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dimitrić Marković, J.M., Milenković, D., Amić, D. et al. Energy requirements of the reactions of kaempferol and selected radical species in different media: towards the prediction of the possible radical scavenging mechanisms. Struct Chem 25, 1795–1804 (2014). https://doi.org/10.1007/s11224-014-0453-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0453-z