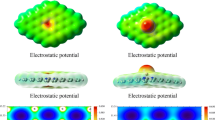
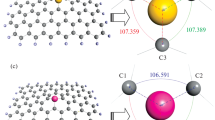
Abstract
Graphene is an important material in adsorption processes because of its high surface. In this work, the interactions between graphene (G), S-doped graphene (SG), and 2S-doped graphene (2SG) with eight small molecules including molecular halogens, CH3OH, CH3SH, H2O, and H2S were studied using density functional theory calculations. The adsorption energies showed that the SG was the best adsorbent, fluorine was the best adsorbate, and all molecular halogens were adsorbed on graphenes better than the other molecules. Most adsorption processes in the gas phase were exothermic with small positive ΔG ads. Moreover, the solvent effect on the adsorption process was examined and all ΔH ads and ΔG ads values for adsorption processes tended to be more negative in all solvents. Therefore, most adsorption processes in the solvents were thermodynamically favorable. The second order perturbation energies obtained from NBO calculations confirmed that the interactions between molecular halogens and our molecules had more strength than those of other molecules. The Laplacian of ρ values obtained from the AIM calculations indicated that the type of interaction in all our complexes was one of closed shell interaction. The MO results and DOS plots also revealed that sulfur doping could increase the conductivity of graphene and this conductivity was enhanced more when they interacted with molecular halogens.


Similar content being viewed by others
References
Xie L, Wang Z, Mao H, Wang R, Ding M, Wang Y, zyilmaz B, Loh KP, Wee A, Chen W (2011) Appl Phys Lett 99:12112–12113
Karlický F, Zbořil R, Otyepka M (2012) J Chem Phys 137:34709–34712
Rani P, Jindal VK (2013) RSC Adv 3:802–812
Castro EV, Novoselov KS, Morozov SV, Peres NMR, Lopes-dosSantos JMB, Nilsson J, Guinea FA, Geim K, Castro-Neto AH (2007) Phys Rev Lett 99:216802–216805
Yang X, Wang Y, Huang XV, Mac Huang Y, Yang R, Duan H, Chen Y (2011) J Mater Chem 21:3448–3454
Kidambi PR, Ducati C, Dlubak B, Gardiner D, Weatherup RS, Martin M, Seneor P, Coles H, Hofmann S (2012) J Phys Chem C 116:22492–22501
Lia W, Magnusona C, Venugopalb A, Ana J, Won Suka J, Hana B, Borysiakc M, Caia W, Velamakannia A, Zhua Y, Fud LM, Vogelb E, Voelkld E, Colomboe LS, Ruoffa R (2010) Nano Lett 10:4328–4334
Li P, You Z, Haugstad G, Cui T (2011) Appl Phys Lett 98:1–253105
Kyle A, Ritter KA, Lyding JW (2008) Nanotechnology 19:15704–15710
Jiao LY, Zhang L, Wang XR, Diankov G, Dai HJ (2009) Narrow Nat 458:877–880
Kosynkin DV, Higginbotham AL, Sinitskii A, Lomeda JR, Dimiev A, Price BK, Tour JM (2009) Nature 458:872–876
Shi G, Ding Y, Fang HJ (2012) Comput Chem 33:1328–1337
Hernández JM, Anota EC, Romero MT, Melchor MG, Cocoletzi GH (2012) J Mol Model 18:3857–3866
Wood BC, Bhide SY, Dutta D, Kandagal VS, Pathak AD, Punnathanam SN, Ayappa KG, Narasimhan S (2012) J Chem Phys 137:54702–54708
Anota EC, Juárez AR, Castro M, Cocoletzi HA (2013) J Mol Model 19:321–328
Kishi H, Tani M, Sakaue M, Nakanishi H, Kasai H (2012) J Vac Soc Jap 55:198–203
Ayala IG, Cordero NA (2012) J Nanopart Res 14:1071
Rudenko AN, Keil FJ, Katsnelson MI, Lichtenstein AI (2012) Phys Rev B 86:075422
Ambrosetti A, Silvestrelli PL (2011) J Phys Chem C 115:3695–3702
Mirzaei M, Yousefi M (2012) Superlattice Microstruct 52:612–617
Wang Y, Shao Y, Matson DW, Li J, Lin Y (2010) ACS Nano 4:1790–1798
Rastegar SF, Peyghan AA, Hadipour NL (2013) Appl Surf Sci 256:412–417
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick; DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Andres JL, Head-Gordon M, Replogle ES, Pople JA (2009) Gaussian 09 Revision A1 Gaussian Inc Wallingford CT
Becke AD (1993) J Chem Phys 98:5648–5654
Lee TC, Yang WT, Parr RG (1988) Phys Rev B 37:785–789
Andersson MP, Uvdal P (2005) J Phys Chem A 109:2937–2941
Mietrus S, Scrocco E (1981) J Chem Phys 55:117–122
Glendening ED, Reed AE, Carpenter JE, Weinhold F, NBO Version 31
Bader RFW (1990) Atoms in molecules a quantum theory. Oxford University Press, New York
O’Boyle NM, Tenderholt AL, Langner KM (2008) J Comp Chem 29:839–845
Tavakol H, Sabzyan H (2011) J Phys Org Chem 24:414–422
Tavakol H (2011) Mol Simul 37:1113–1121
Tavakol H (2011) Int J Quantum Chem 111:3717–3724
Tavakol H (2010) J Mol Struct 954:16–21
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tavakol, H., Mollaei-Renani, A. DFT, AIM, and NBO study of the interaction of simple and sulfur-doped graphenes with molecular halogens, CH3OH, CH3SH, H2O, and H2S. Struct Chem 25, 1659–1667 (2014). https://doi.org/10.1007/s11224-014-0446-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0446-y