Abstract
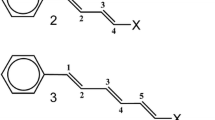
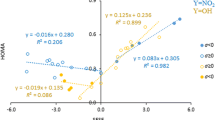
The transmission of long-range polar effects (field effects) across the diamantane cage has been investigated by analyzing the small structural changes induced by a variable substituent X in the phenyl group of 9-substituted 4-phenyldiamantane derivatives. The structures of many such molecules with charged or dipolar substituents have been determined from quantum chemical calculations at the HF/6-31G* and B3LYP/6-311++G** levels of theory. Comparison with the results of a similar study carried out on 4-substituted 1-phenylbicyclo[2.2.2]octane derivatives, where the distance between probe and substituent is substantially smaller, shows that the ability of the diamantane framework to transmit field effects is 45 % of that of the bicyclo[2.2.2]octane framework when X is a dipolar, uncharged substituent. This figure increases to 59 % in the case of charged groups. The structural results support the idea that the field effect of a dipolar substituent attenuates more rapidly with distance than that of a charged group. This makes it impossible to construct a single, universal scale of field parameters including both dipolar and charged groups. A single scale can only be set up for a fixed separation between substituent and probe. The presence of the variable substituent X has a pronounced effect on the geometry of the diamantane cage. The nonbonded distance between the bridgehead carbons C4 and C9 spans an interval about 0.20 Å wide and correlates quite well with the mean value of the three cage angles at C9. These geometric changes are closely similar to those of the corresponding parameters in 4-substituted 1-phenylbicyclo[2.2.2]octane derivatives. The concerted structural variation of the polycyclic cage is controlled primarily by the group electronegativity of X and does not correlate with the much smaller structural variation of the phenyl probe.







Similar content being viewed by others
References
Stock LM (1972) J Chem Educ 49:400–404
Exner O, Fiedler P (1980) Collect Czechoslov Chem Commun 45:1251–1268
Bowden K, Grubs EJ (1993) Prog Phys Org Chem 19:183–224
Exner O, Friedl Z (1993) Prog Phys Org Chem 19:259–294
Koppel IA, Mishima M, Stock LM, Taft RW, Topsom RD (1993) J Phys Org Chem 6:685–689
Bowden K, Grubs EJ (1996) Chem Soc Rev 25:171–177
Adcock W, Trout NA (1999) Chem Rev 99:1415–1435
Exner O, Böhm S (2002) Chem Eur J 8:5147–5152
Wiberg KB (2002) J Org Chem 67:1613–1617
Adcock W, Baran Y, Filippi A, Speranza M, Trout NA (2005) J Org Chem 70:1029–1034
Adcock W (2009) J Phys Org Chem 22:1065–1069
Campanelli AR, Domenicano A, Ramondo F (2006) J Phys Chem A 110:10122–10129
Campanelli AR, Domenicano A, Piacente G, Ramondo F (2010) J Phys Chem A 114:5162–5170
Campanelli AR, Domenicano A, Ramondo F (2011) Struct Chem 22:449–457
Gund TM, Osawa E, Williams VZ Jr, Schleyer PvR (1974) J Org Chem 39:2979–2987 (and references quoted therein)
Cupas C, Schleyer PvR, Trecker DJ (1965) J Am Chem Soc 87:917–918
Karle IL, Karle J (1965) J Am Chem Soc 87:918–920
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision C.02. Gaussian, Inc., Wallingford
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, revision C.01. Gaussian, Inc., Wallingford
Campanelli AR, Domenicano A, Ramondo F (2003) J Phys Chem A 107:6429–6440
Campanelli AR, Domenicano A, Ramondo F, Hargittai I (2004) J Phys Chem A 108:4940–4948
Campanelli AR, Domenicano A, Macchiagodena M, Ramondo F (2011) Struct Chem 22:1131–1141
Campanelli AR, Domenicano A, Ramondo F (2012) J Phys Chem A 116:8209–8217
Campanelli AR, Domenicano A (2013) Struct Chem 24:867–876
Campanelli AR (2013) Struct Chem 24:859–866
Laidler KJ, Meiser JH, Sanctuary BC (2003) Physical chemistry, 4th edn. Houghton Mifflin Company, Boston, p 911
Exner O (1978) A critical compilation of substituent constants. In: Chapman NB, Shorter J (eds) Correlation analysis in chemistry: recent advances. Plenum Press, New York, pp 439–540
Hansch C, Leo A, Taft RW (1991) Chem Rev 91:165–195
Cherkasov AR, Galkin VI, Cherkasov RA (1996) Russ Chem Rev 65:641–656
Walsh AD (1947) Discuss Faraday Soc 2:18–25
Bent HA (1961) Chem Rev 61:275–311
Bent HA (1961) J Inorg Nucl Chem 19:43–50
Gillespie RJ (1972) Molecular geometry. Van Nostrand Reinhold, London
Gillespie RJ, Hargittai I (1991) The VSEPR model of molecular geometry. Allyn and Bacon, Boston (2012, Dover, Mineola)
Domenicano A, Vaciago A, Coulson CA (1975) Acta Crystallogr B31:221–234
Acknowledgments
This work was supported by the CASPUR Supercomputing Center, Rome, with a Standard HPC Grant 2012 (“A combined X-ray absorption spectroscopy, molecular dynamics simulations, and quantum mechanics calculation procedure for the structural characterization of ill-defined systems”) and by the CINECA Supercomputing Center, Bologna, with Project IsC10_DYNGEO_E.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Campanelli, A.R., Domenicano, A. Transmission of electronic substituent effects through cage polycyclic alkanes: a computational study of diamantane derivatives based on structural variation. Struct Chem 25, 691–698 (2014). https://doi.org/10.1007/s11224-014-0400-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0400-z