Abstract
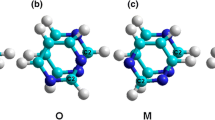
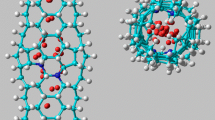
The hydrogen adsorption energies for nitrogen-containing carbon nanotubes (N-CNTs) and for bare carbon nanotubes were calculated using the density functional theory methods at the B3LYP/6–31-G(d) level, including dispersion force corrections. The N-CNTs were finite saturated and non-saturated single-walled carbon nanotubes that contained one or more pyrimidine units, the relative positions of which defined the different configurations of the nanotube. The chemisorption of atomic hydrogen to a full exocyclic monolayer of zigzag, armchair, and chiral N-CNTs was studied as a function of the structural parameters. Zigzag N-CNTs of any configuration, with a larger number of nitrogen atoms, a small diameter and a small length, are more reactive compared to chiral and armchair N-CNTs. The presence of nitrogen in the carbon nanotubes enhances their reactivity to chemisorb atomic hydrogen, showing exothermic energy values. In contrast, the physisorption of molecular hydrogen was endothermic for most of the studied saturated N-CNTs, even when including corrections for van der Waals interactions. The endothermicity was greatest for zigzag nanotubes, then decreased for chiral nanotubes and decreased again for armchair nanotubes. In general, the endothermicity decreased for longer nanotubes, which have larger diameters, and a small number of nitrogen atoms. The results of this study suggest that, with saturated bare carbon nanotubes, saturated, and unsaturated N-CNTs could potentially have a higher capacity as hydrogen-storage media than the corresponding unsaturated carbon nanotubes.








Similar content being viewed by others
References
De Volder MFL, Tawfick SH, Baughman RH, Hart AJ (2013) Carbon nanotubes: present and future commercial applications. Science 339:535–539
Bareket-Keren L, Hanein Y (2013) Carbon nanotube-based multielectrode arrays for neuronal interfacing: progress and prospects. Front Neural Circuits 6:122
Bianco S (2011) Carbon nanotubes. From research to applications. Intech, Croatia
Marulanda JM (2010) Carbon nanotubes. In-The, India
Kovalev V, Yakunchikov A, Li F (2011) Simulation of hydrogen adsorption in carbon nanotube arrays. Acta Astronaut 68:681–685
Yao Y (2010) In: Marulanda JM (ed) Carbon nanotubes. In-The, India
Targets for onboard hydrogen storage systems for light-duty vehicles http://www.eere.energy.gov/hydrogenandfuelcells/storage/pdfs/targets_onboard_hydro_storage_explanation.pdf. Accessed 12 Sept 2013 http://www1.eere.energy.gov/hydrogenandfuelcells/storage/current_technology.html. Accessed 12 Sept 2013
Dillon AC, Jones KM, Bekkedahl TA, Kiang CH, Bethune DS, Heben MJ (1997) Storage of hydrogen in single-walled carbon nanotubes. Nature 386(6623):377–379
Zhang G, Qi P, Wang X, Lu Y, Mann D, Li X, Dai H (2006) Hydrogenation and hydrocarbonation and etching of single-walled carbon nanotubes. J Am Chem Soc 128:6026–6027
Baughman RH, Zakhidov AA, de Heer WA (2002) Carbon nanotubes-the route toward applications. Science 297(5582):787–792
Bilic A, Gale JD (2008) Chemisorption of molecular hydrogen on carbon nanotubes: a route to effective hydrogen storage? J Phys Chem C 112:12568–12575
Dinadayalane TC, Kaczmarek A, Lukaszewicz J, Leszczynski J (2007) Chemisorption of hydrogen atoms on the sidewalls of armchair single-walled carbon nanotubes. J Phys Chem C 111:7376–7383
Kaczmarek A, Dinadayalane TC, Lukaszewicz J, Leszczynski J (2007) Effect of tube length on the chemisorptions of one and two hydrogen atoms on the sidewalls of (3,3) and (4,4) single-walled carbon nanotubes: a theoretical study. Int J Quantum Chem 107:2211–2219
Alonso JA, Arellano JS, Molina LM, Rubio A, López MJ (2004) Interaction of molecular and atomic hydrogen with single-wall carbon nanotubes. IEEE Trans Nanotech 3:304–310
Orimo S, Züttel A, Schlapbach L, Majer G, Fukunaga T, Fujii H (2003) Hydrogen interaction with carbon nanostructures: current situation and future prospects. J Alloys Compd 356–357:716–719
Becher M, Haluska M, Hirscher M, Quintel A, Skakalova V, Dettlaff-Weglikovska U, Chen X, Hulman M, Choi Y, Roth S, Meregalli V, Parrinello M, Ströbel R, Jörissen L, Kappes MM, Fink J, Züttel A, Stepanek I, Bernier P (2003) Hydrogen storage in carbon nanotubes. C. R. Physique 4:1055–1062
Ross DK (2006) Hydrogen storage: the major technological barrier to the development of hydrogen fuel cell cars. Vacuum 80:1084–1089
Charlier JC (2002) Defects in carbon nanotubes. Acc Chem Res 35:1063–1069
Czerw R, Terrones M, Charlier JC, Blase X, Foley B, Kamalakaran R, Grobert N, Terrones H, Ajayan PM, Blau W, Tekleab D, Rühle M, Carroll DL (2001) Identification of electron donor states in N-doped carbon nanotubes. Nano Lett 1:457–460
Terrones M (2007) Synthesis toxicity and applications of doped carbon nanotubes. Acta Microsc 16(1–2):33–34
Hu X, Zhou Z, Lin Q, Wu Y, Zhang Z (2011) High reactivity of metal-free nitrogen-doped carbon nanotube for the C-H activation. Chem Phys Lett 503:287–291
Xiong W, Du F, Liu Y, Perez A Jr, Supp M, Ramakrishnan TS, Dai L, Jiang L (2010) 3-D carbon nanotube structures used as high performance catalyst for oxygen reduction reaction. J Am Chem Soc 132:15839–15841
Gong KP, Du ZH, Xia ZH, Durstock M, Dai LM (2009) Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323:760–764
Zhang Y, Wen B, Song XY, Li TJ (2010) Synthesis and bonding properties of carbon nanotubes with different nitrogen contents. Acta Phys Sin 59(5):3583–3588
Yang SH, Shin WH, Kang JK (2008) The nature of graphite- and pyridinelike nitrogen configurations in carbon nitride nanotubes: dependence on diameter and helicity. Small 4:437–441
Zhong Z, Lee GI, Mo CB, Hong SH, Kang JK (2007) Tailored field-emission property of patterned carbon nitride nanotubes by a selective doping of substitutional N(sN) and pyridine-like N(pN) atoms. Chem Mater 19:2918–2920
Trasobares S, Stephan O, Colliex C, Hsu WK, Kroto HW, Walton DRM (2002) Compartmentalized CNx nanotubes: chemistry morphology and growth. J Chem Phys 116:8966–8972
Zhang ZY, Cho K (2007) Ab initio study of hydrogen interaction with pure and nitrogen-doped carbon nanotubes. Phys Rev B 75(7):075420
Zhou Z, Gao XP, Yan J, Song DY (2006) Doping effects of B and N on hydrogen adsorption in single-walled carbon nanotubes through density functional calculations. Carbon 44:939–947
Rangel E, Ruiz-Chavarria G, Magana LF, Arellano JS (2009) Hydrogen adsorption on N-decorated single wall carbon nanotubes. Phys Lett A 373:2588–2591
Oh KS, Kim DH, Park S, Lee JS, Kwon O, Choi YK (2008) Movement of hydrogen molecules in pristine hydrogenated and nitrogen-doped single-walled carbon nanotubes. Mol Simul 34:1245–1252
Contreras ML, Rozas R (2011) In: Bianco S (ed) Carbon nanotubes. From research to applications. Intech, Croatia. http://www.intech.open.com/books/carbon-nanotubes-from-research-to-applications/nitrogen-containing-carbon-nanotubes-a-theoretical-approach
Contreras ML, Avila D, Alvarez J, Rozas R (2012) Computational algorithms for a fast building of 3-D carbon nanotube models having different defects. J Mol Graph Mod 38:389–395
HyperChem release 7.5 Hypercube Inc 1115 NW 4th Street Gainesville Florida 32601 USA
Jaguar version 7.8 Schrödinger LLC New York NY 2011
Becke AD (1993) Density-functional thermochemistry. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the colle-salvetti correlation-energy formula into a functional of the electron-density. Phys Rev B 37:785–789
Ahmadi A, Beheshtian J, Hadipour NL (2011) Chemisorption of NH3 at the open ends of boron nitride nanotubes: a DFT study. Struct Chem 22:183–188
Contreras ML, Avila D, Alvarez J, Rozas R (2010) Exploring the structural and electronic properties of nitrogen-containing exohydrogenated carbon nanotubes: a quantum chemistry study. Struct Chem 21(3):573–581
Beheshtian J, Peyghan AA, Bagheri Z (2013) Hydrogen dissociation on diene-functionalized carbon nanotubes. J Mol Model 19:255–261
Felice RD, Calzolari A, Varsano D, Rubio A (2005) Electronic structure calculations for nanomolecular systems. Lect Notes Phys 680:77
Dreizler RM, Gross EKU (1990) Density functional theory, an approach to the quantum many body problem. Springer, Berlin
Van de Walle CG (2008) Computational studies of conductivity in wide-band-gap semiconductors and oxides. J Phys: Condens Matter 20:064230
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132(15):154104
Neese F (2012) The ORCA program system. WIREs Comput Mol Sci 2(1):73–78
Vydrov OA, Van Voorhis TJ (2010) Nonlocal van der Waals density functional: the simpler the better. Chem Phys 133:244103 (9 pp)
Hujo W, Grimme S (2011) Performance of the van der Waals density functional VV10 and (hybrid)GGA variants for thermochemistry and noncovalent interactions. J Chem Theory Comput 7(12):3866–3871
Zhao M, Xia Y, Lewis JP, Zhang R (2003) First-principles calculations for nitrogen-containing single-walled carbon nanotubes. J Appl Phys 94(4):2398–2402
Tada K, Furuya S, Watanabe K (2001) Ab initio study of hydrogen adsorption to single-walled carbon nanotubes. Phys Rev B 63:155405 (4 pp)
Shamina EN, Lebedev NG (2012) The chiral effect of adsorption of univalent atoms and diatomic molecules on the surface of carbon nanotubes. Russ J Phys Chem B 6:448–454
Yang FH, Lachawiec AJ Jr, Yang RT (2006) Adsorption of spillover hydrogen atoms on single-wall carbon nanotubes. J Phys Chem B 110:6236–6244
Gayathri V, Geetha R (2007) Hydrogen adsorption in defected carbon nanotubes. Adsorpt 13:53–59
Singh AK, Yakobson BI (2012) First principles calculations of H-storage in sorption materials. J Mater Sci 47:7356–7366
Lochan RC, Head-Gordon M (2006) Computational studies of molecular hydrogen binding affinities: the role of dispersion forces electrostatics and orbital interactions. Phys Chem Chem Phys 8:1357–1370
Li J, Furuta T, Goto H, Ohashi T, Fujiwara Y, Yip S (2003) Theoretical evaluation of hydrogen storage capacity in pure carbon nanostructures. J Chem Phys 119:2376
Bhatia SK, Myers AL (2006) Optimum conditions for adsorptive storage. Langmuir 22:1688–1700
DiLabio GA, Koleini M, Torres E (2013) Extension of the B3LYP–dispersion-correcting potential approach to the accurate treatment of both inter- and intra-molecular interactions. Theor Chem Acc 132:1389
Acknowledgments
This work was partially supported by the Direction of Scientific and Technological Research DICYT-USACH Project Nr. 061342CF and by the Sociedad de Desarrollo Tecnológico SDT-USACH project Nr. CIA 2981. In addition, the central cluster of the Faculty of Chemistry and Biology and the VRID of the University of Santiago de Chile are acknowledged for allocating computational resources.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Contreras, M.L., Cortés-Arriagada, D., Villarroel, I. et al. Evaluating the hydrogen chemisorption and physisorption energies for nitrogen-containing single-walled carbon nanotubes with different chiralities: a density functional theory study. Struct Chem 25, 1045–1056 (2014). https://doi.org/10.1007/s11224-013-0377-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0377-z