Abstract
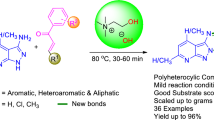
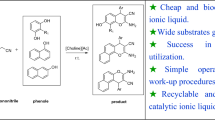
An efficient and straightforward approach to the synthesis of 2,3-dihydroquinazolin-4(1H)-one from 2-aminobenzamide and carbonyl compounds (aldehydes and ketones) using biocompatible choline sulfate-based acidic ionic liquid as a cheap and readily available catalyst in water has been developed. Various 2,3-dihydroquinazolin-4(1H)-one have been prepared using low-cost and environmental friendly solvent and catalyst in good to excellent yields in a shorter reaction time. The choline sulfate catalyst was prepared using a simple method from readily available starting material and was confirmed by 1H NMR, FTIR, and TGA. The ease of the product separation without organic solvent and column chromatography and the reusability of the acidic ionic liquid catalyst makes this method economically affordable for large-scale synthesis.






Similar content being viewed by others
References
R.A. Sheldon, J.P.M. Sanders, Catal. Today 239, 3 (2015)
G.L. Khatik, A. Kumar, A.K. Chakraborti, Org. Lett. 8, 2433 (2006)
A.S. Amarasekara, Chem. Rev. 116, 6133 (2016)
R.A. Sheldon, Chem. Soc. Rev. 41, 1437 (2012)
P. Wang, F.-P. Ma, Z.-H. Zhang, J. Mol. Liq. 198, 259 (2014)
N. Azizi, B. Mirmashhori, M.R. Saidi, Catal. Commun. 8, 2198 (2007)
P.M. Pawar, K.J. Jarag, G.S. Shankarling, Green Chem. 13, 2130 (2011)
P. Liu, J.W. Hao, L.P. Mo, Z.H. Zhang, RSC Adv. 5, 48675 (2015)
J. Rivoal, A.D. Hanson, Plant Physiol. 106, 1187 (1994)
P. Nissen, A.A. Benson, Science 134, 1759 (1961)
M.A. Chaudhari, J.B. Gujar, D.S. Kawade, P.V. Shinde, M.S. Shingare, Res. Chem. Intermed. 44, 10027 (2015)
S.L. Schreiber, Nat. Chem. Biol. 1, 64 (2005)
P.N. Kalaria, S.P. Satasia, J.R. Avalani, D.K. Raval, Eur. J. Med. Chem. 83, 655 (2014)
S.P. Satasia, P.N. Kalaria, J.R. Avalani, D.K. Raval, Tetrahedron 70, 5763 (2014)
J.F. Wolfe, T.L. Rathman, M.C. Sleevi, J.A. Campbell, T.D. Greenwood, J. Med. Chem. 33, 161 (1990)
X.-F. Wu, S. Oschatz, A. Block, A. Spannenberg, P. Langer, Org. Biomol. Chem. 12, 1865 (2014)
N. Azizi, M.R. Saidi, Phosphorus Sulf. Silicon Relat. Elem. 178, 1255 (2003)
A.V.D. Rao, B.P. Vykunteswararao, T. Bhaskarkumar, N.R. Jogdand, D. Kalita, J.K.D. Lilakar, V. Siddaiah, P.D. Sanasi, A. Raghunadh, Tetrahedron Lett. 56, 4714 (2015)
J. Wang, Y. Zong, R. Fu, Y. Niu, G. Yue, Z. Quan, X. Wang, Y. Pan, Ultrason. Sonochem. 21, 29 (2014)
R. Sharma, A. K. Pandey, P. M. S. Chauhan, Synlett 23, 2209 (2012)
H. Hikawa, Y. Ino, H. Suzuki, Y. Yokoyama, J. Org. Chem. 77, 7046 (2012)
S. Hakim, K. Kon, A.S. Touchy, K.I. Shimizu, Catal Sci. Technol. 4, 1716 (2014)
R.A. Bunce, B. Nammalwar, J. Heterocycl. Chem. 48, 991 (2011)
G. Cai, X. Xu, Z. Li, W.P. Weber, P. Lu, J. Heterocycl. Chem. 39, 1271 (2002)
W. Su, B. Yang, Aust. J. Chem. 55, 695 (2002)
S. Tarannum, N. Ahmed, Z.N. Siddiqui, Catal. Commun. 66, 60 (2015)
D. Kumar, P.S. Jadhavar, M. Nautiyal, H. Sharma, P.K. Meena, L. Adane, S. Pancholia, A.K. Chakraborti, RSC Adv. 5, 30819 (2015)
S. Guo, Y. Li, L. Tao, W. Zhang, X. Fan, RSC Adv. 4, 59289 (2014)
M. Boomhoff, R. Ukis, C. Schneider, J. Org. Chem. 80, 8236 (2015)
J.K. Laha, K.S.S. Tummalapalli, A. Nair, N. Patel, J. Org. Chem. 80, 11351 (2015)
H.R. Lobo, B.S. Singh, G.S. Shankarling, Catal. Commun. 27, 179 (2012)
T.B. Nguyen, L. Ermolenko, A. Al-Mourabit, Green Chem. 15, 2713 (2013)
M. Prakash, S. Jayakumar, V. Kesavan, Synthesis 45, 2265 (2013)
Y. Liu, L. Lu, Y.-J. Zhou, X.-S. Wang, Res. Chem. Intermed. 40, 2823 (2014)
Z.H. Zhang, X.N. Zhang, L.P. Mo, Y.X. Li, F.P. Ma, Green Chem. 14, 1502 (2012)
L.Y. Zeng, C. Cai, J. Heterocycl. Chem. 47, 1035 (2010)
J. Wu, X. Du, J. Ma, Y. Zhang, Q. Shi, L. Luo, B. Song, S. Yang, D. Hu, Green Chem. 16, 3210 (2014)
A.K. Sanap, G.S. Shankarling, New J. Chem. 39, 206 (2015)
X.N. Zhao, G.F. Hu, M. Tang, T.T. Shi, X.L. Guo, T.T. Li, Z.H. Zhang, RSC Adv. 4, 51089 (2014)
S. Santra, M. Rahman, A. Roy, A. Majee, A. Hajra, Catal. Commun. 49, 52 (2014)
B. Zhang, L. Shi, R. Guo, Catal. Lett. 145, 1718 (2015)
A. Taheri, B. Lai, C. Cheng, Y. Gu, Green Chem. 17, 812 (2015)
J. Wang, M.M. Zhang, X.S. Wang, Res. Chem. Intermed. (2016). doi:10.1007/s11164-016-2807-1
N. Azizi, E. Batebi, Catal. Sci. Technol. 2, 2445 (2012)
N. Azizi, E. Gholibeglo, RSC Adv. 2, 7413 (2012)
N. Azizi, S. Dezfooli, M.M. Hashemi, J. Mol. Liq. 194, 62 (2014)
P.N. Borase, P.B. Thale, G.S. Shankarling, RSC Adv. 6, 63078–63083 (2016)
F. Tamaddon, M.T. KazemiVarnamkhasti, Synlett 27, 2510 (2016)
Acknowledgements
Financial support of this work by Chemistry and Chemical Engineering Research Center of Iran is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azizi, N., Shirdel, F. Cholinesulfuric acid ionic liquid catalyzed an eco-friendly synthesis of 2,3-dihydroquinazolin-4(1H)-one in aqueous media. Res Chem Intermed 43, 3873–3882 (2017). https://doi.org/10.1007/s11164-016-2849-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2849-4