Abstract
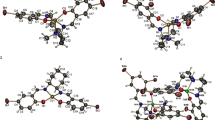
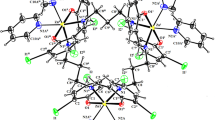
Two Schiff bases, HL1 and HL2, were synthesized in two different reactions involving 2-hydroxynaphthaldehyde with 2-amino-6-methylbenzothiazole and 2-amino-6-florobenzothiazole respectively. Copper(II) and nickel(II) complexes of the Schiff bases were subsequently prepared in 1:1 metal-to-ligand stoichiometric reactions. The compounds were characterized extensively by 1H NMR, 13C NMR, Dept-90, UV–Vis, and IR spectroscopic techniques, magnetic susceptibility, TGA, DTG, and molar conductivity analysis. The spectroscopic results confirm bidentate nature of the Schiff bases and a four coordinate geometry for all the complexes: [CuL1ClH2O], [NiL1ClH2O], [Cu(L2)2], and [NiL2ClH2O]. Quantum chemical studies gave fully optimized geometries of the Schiff bases and metal complexes using the 6-31+g(d,p) basis set. The compounds were studied for their in vitro antibacterial activities against some selected Gram-positive and Gram-negative bacteria, using agar well diffusion. The metal complexes exhibited better antibacterial activities compared to the free ligand due to the effects of chelation, which improved the lipophilicity. Furthermore, the antioxidant potentials of the compounds were ascertained using DPPH radical scavenging and ferrous chelating assay. The copper complexes had the best antioxidant properties of all the tested compounds. The results of the biological analysis were in agreement with the theoretical data from quantum chemical calculations. The study presented biologically active coordination compounds with benzothiazole moiety that could be used as compounds of interest in the drug discovery processes.






Similar content being viewed by others
References
N.B. Patel, F.M. Shaikh, New 4-thiazolidinones of nicotinic acid with 2-amino-6-methylbenzothiazole and their biological activity. Sci. Pharm. 78, 753–765 (2010)
R. Abdul (2005) Synthesis and Biological Studies of Some Schiff bases Compounds and Their Transition Metal Complexes. A Ph.D. thesis submitted to the Bahuaddin Zakariya University, Multan Pakistan
P. Chaudhary, P. Sharma, A. Sharma, J. Varshney, Recent advances in pharmacological activity of benzothiazole derivatives. Int. J. Curr. Pharm. Res. 4, 5–11 (2010)
A. Rana, N. Siddiqui, S.A. Khan, Benzothiazoles: a new profile of biological activities. Int. J. Curr. Pharm. Res. 69, 10–17 (2007)
J. Malik, F.V. Manvi, B.K. Nanjwade, P. Purohit, New 2-amino substituted benzothiazoles: a new profile of biological activities. J. Pharm. Res. 11, 1687–1690 (2009)
J.K. Malik, F.V. Manvi, B.K. Nanjwade, S. Singh, P. Purohit, Review of the 2-amino substituted benzothiazoles: different methods of the synthesis. Der Pharm. Lett. 2, 347–359 (2010)
J.H. Block, J.H. Beale, Wilson and Gisvold, Textbook of Organic, Medicinal and Pharmaceutical Chemistry, 10th edn. (Lipincott William & Wilkins, New York, 1998)
L.S. Khokra, K. Arora, H. Mehta, A. Aggarwal, M. Yadav, Essential oil composition and antibacterial studies of Vitex negundo Linn. extracts. Int. J. Pharm. Sci. Res. 2, 1356–1377 (2011)
H.A. Bhuva, S.G. Kini, Synthesis, anticancer activity and docking of some substituted benzothiazoles as tyrosine kinase inhibitors. J. Mol. Graph. Model. 29, 32–37 (2010)
C.G. Mortimer, G. Wells, J.P. Crochard, E.L. Stone, T.D. Bradshaw, M.F.G. Stevens, A.D. Westwell, Antitumor benzothiazoles. 26. 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon and breast cancer cell lines. J. Med. Chem. 49, 179–185 (2006)
S. Nagarjan, G. Crescenzo, D. Getman, H. Lu, J. Sikorski, J. Walker, J. McDonald, K. Houseman, G. Kocan, N. Kishore, P. Mehta, C. Shippy, L. Blystone, Discovery of novel benzothiazolesulfonamides as potent inhibitors of HIV-1 protease. Bioorg. Med. Chem. 11, 4769–4777 (2003)
S. Hout, N. Azas, A. Darque, M. Robin, C. Giorgio, M. Gasquet, J. Galy, P. David, Activity of benzothiazoles and chemical derivatives on Plasmodium falciparum. Parasitology 129, 525–542 (2004)
M. Maharan, S. William, F. Ramzy, A. Sembel, Synthesis and in vitro evaluation of new benzothiazole derivatives as schistosomicidal agents. Molecules 12, 622–623 (2007)
V. Kumar, T.S. Ngaraja, H. Shameer, E. Jayachandran, G.M. Sreenivasa, N-substituted-3-chloro-2-azetidinones: synthesis and characterization of new novel anti-inflammatory agents. J. Pharm. Sci. Res. 2, 83–92 (2009)
S. Hibi, Y. Okamoto, K. Tagami, H. Numata, N. Kobayashi, M. Shinoda, T. Kawahara, M. Murakami, K. Oketani, T. Inoue, H. Shibata, I. Yamatsu, Novel dual inhibitors of 5-lipoxygenase and thromboxane A2 synthetase: synthesis and structure-activity relationships of 3-pyridylmethyl-substituted 2-amino-6-hydroxybenzothiazole derivatives. J. Med. Chem. 37, 3062–3070 (1994)
N. Siddiqui, A. Rana, S. Khan, S. Haque, M. Arshad, S. Ahmed, W. Ahsan, Syntheis and preliminary screening of benzothiazol-2yl-thiadiazole derivatives for anticonvulsant activity. Acta Pharm. 59, 441–451 (2009)
N. Siddiqui, A. Rana, S. Khan, S. Haque, M. Alam, W. Ahsan, M. Arshad, Anticonvulsant and toxicity evaluation of newly synthesized 1-[2-(3,4-disubstitutedphenyl)-3-chloro-4-oxoazetidin-1-yl]-3-(6-substituted-1,3-benzothiazol-2-yl)ureas. Acta Chim. Slov. 59, 462–469 (2009)
A. Nitta, H. Fujii, S. Sakami, Y. Nishimura, T. Ohyama, M. Satoh, J. Nakaki, S. Satoh, C. Inada, H. Kozono, H. Kumagai, M. Shimamura, T. Fukazawa, H. Kawai, (3R)-3-amino-4-(2,4,5-trifluorophenyl)-N-{4-[6-(2-methoxyethoxy)benzothiazol-2-yl]tetrahydropyran-4-yl}butanamide as a potent dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg. Med. Chem. Lett. 18, 5435–5438 (2008)
H. Diaz, R. Molina, R. Andrade, D. Coutino, J. Franco, S. Webster, M. Binniie, S. Estrada-Soto, M.I. Brajas, I.L. Rivera, G.N. Vazquez, Antidiabetic activity of N-(6-substituted-1,3-benzothiazol-2-yl)benzenesulfonamides. Bioorg. Med. Chem. Lett. 18, 2871–2877 (2008)
P. Arora, S. Das, M.S. Ranawat, N. Arora, M.M. Gupta, Synthesis and biological evaluation of some novel chrome-2-one derivatives for antipsychotic activity. J. Chem. Pharm. Res. 2, 317–323 (2010)
S.Y. Shahar, Z.H. Ansari, Synthesis and in vivo diuretic activity of biphenyl benzothiazole-2-carboxamide derivatives. Acta Pol. Pharm. Drug Res. 66, 387–392 (2009)
T.D. Bradshaw, M.S. Chua, H.L. Browne, V. Trapani, E.A. Sausville, M.F.G. Stevens, In vitro evaluation of amino acid prodrugs of novel antitumour 2-(4-amino-3-methylphenyl)benzothiazoles. Br. J. Cancer 86, 1348 (2002)
F. Delmas, A. Avellaneda, C.D. Gioegia, M. Robin, E.D. Clercq, D.P. Timol, J.P. Galy, Synthesis and antileishmanial activity of (1,3-benzothiazol-2-yl) amino-9-(10H)-acridinone derivatives. Eur. J. Med. Chem. 39, 685 (2004)
S.R. Pattan, S.N.N. Babu, J. Angadi, Synthesis and biological activity of 2-amino [5′-(4′-sulphonylbenzylidene)-2,4-thiazolidine dione]-7-(substituted)-6-fluoro benzothiazoles. Indian J. Heterocycl. Chem. 11, 333 (2002)
A.A. Osowole, A.C. Ekennia, B.O. Achugbu, Synthesis, spectroscopic characterization and antibacterial properties of some metal (II) complexes of 2-(6-methoxybenzothiazol-2-ylimino) methyl)-4-nitrophenol. Res. Rev. J. Pharm. Anal. 2, 1–5 (2013)
H.R.I. Tomi, J.H. Tomma, A.H.R. Al-Daraji, A.H. Al-Dujaili, Synthesis, characterization and comparative study the microbial activity of some heterocyclic compounds containing oxazole and benzothiazole moieties. J. Saudi Chem. Soc. 2012, 213–232 (2012)
H. Mahmood-ul, Z.H. Chohan, C.T. Supuran, Antibacterial Zn(II) compounds of Schiff bases derived from some benzothiazoles. Main Group Met. Chem. 25, 291 (2002)
S.P. Vartale, D.B. Kadam, N.K. Halikar, Synthesis of novel 4-thiazolidinone derivatives incorporated with benzothiazole and its antimicrobial activity. Der Pharma Chem. 3, 213–223 (2011)
R. Ali, N. Siddiqui, Biological aspects of emerging benzothiazoles: a short review. J. Chem 2013, 12 (2013). doi:10.1155/2013/345198
R. Caputo, M.L. Calabro, N. Micale, Synthesis and biological evaluation of new 2-amino-6-(trifluoromethoxy)benzoxazole derivatives, analogues of riluzole. Med. Chem. Res. 21, 2644–2651 (2012)
A.C. Ekennia, D.C. Onwudiwe, A.A. Osowole, Spectral, thermal stability ans antibacterial studies of copper complexes of N-methyl-N-phenyl dithiocarbamate. J. Sulfur Chem. 36, 96–104 (2015)
G.A. Kolawole, A.A. Osowole, Synthesis and characterization of some metal (II) complexes of isomeric unsymmetrical Schiff bases and their adducts with triphenylphosphine. J. Coord. Chem. 62, 1437–1444 (2009)
A.A. Osowole, R. Kempe, R. Schobert, Synthesis, spectral, thermal, in-vitro antibacterial and anticancer activities of some metal (II) complexes of 3-(-1-(4-methoxy-6-methyl)-2-pyrimidinylimino)methyl-2-napthol. Int. Res. J. Pure Appl. Chem. 2, 105–129 (2012)
A.C. Ekennia, D.C. Onwudiwe, C. Ume, E.E. Ebenso, Mixed ligand complexes of N-methyl-N-phenyldithiocarbamate: synthesis, characterisation, antifungal activity and solvent extraction studies of the ligand. Bioinorg. Chem. Appl. (2015). doi:10.1155/2015/913424
A.D. Becke, Density-functional exchange-energy approximation with correct asymptotic behaviour. Phys. Rev. A 38, 3098–3100 (1988)
C. Lee, W. Yang, R.G. Parr, Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988)
X. Xu, W.A. Goddard, The X3LYP extended density functional for accurate descriptions of nonbond interactions, spin states, and thermochemical properties. Proc. Natl. Acad. Sci. 101, 2673–2677 (2003)
I. Georgieva, N. Trendafilova, Bonding analyses, formation energies, and vibrational properties of M-R2dtc complexes (M = Ag (I), Ni (II), Cu (II), or Zn (II)). J. Phys. Chem. A 111, 13075–13087 (2007)
L. Chen, T. Liu, C. Ma, Metal complexation and biodegradation of EDTA and S, S-EDDS: a density functional theory study. J. Phys. Chem. A 114, 443–454 (2007)
Y. Niu, S. Feng, Y. Ding, R. Qu, D. Wang, J. Han, Theoretical investigation on sulfur-containing chelating resin-divalent metal complexes. Int. J. Quantum Chem. 110, 1982–1993 (2010)
S.I. Gorelsky, L. Basumallick, J. Vura-Weis, R. Sarangi, K.O. Hodgson, B. Hedman, K. Fujisawa, E.I. Solomon, Spectroscopic and DFT investigation of [M{HB(3,5-iPr2pz)3}(SC6F5)] (M = Mn, Fe Co, Ni, Cu, and Zn) model complexes: periodic trends in metal–thiolate bonding. Inorg. Chem. 44, 4947–4960 (2005)
T. Marino, M. Toscano, N. Russo, A. Grand, Structural and electronic characterization of the complexes obtained by the interaction between bare and hydrated first-row transition-metal ions (Mn 2 + , Fe 2 + , Co 2 + , Ni 2 + , Cu 2 + , Zn 2 +) and glycine. J. Phys. Chem. B 110, 24666–24673 (2006)
R. Terreux, M. Domard, C. Viton, A. Domard, Interactions study between the copper II ion and constitutive elements of chitosan structure by DFT calculation. Biomacromolecules 7, 31–37 (2006)
T.H. Dunning, P.Y. Hay, in Modern Theoretical Chemistry, 3rd edn., ed. by H.F. Schaefer (Plenum, New York, 1976)
P.J. Hay, W.R. Wadt, Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82, 270–283 (1985)
W.R. Wadt, P.J. Hay, Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 82, 284–298 (1985)
P.J. Hay, W.R. Wadt, Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 82, 299–310 (1985)
M.Y. Combariza, R.W. Vachet, J. Phys. Chem. A 108, 757–1763 (2004)
M.A. Carvajal, J.J. Novoa, S. Alvarez, Choice of coordination number in d10 complexes of group 11 metals. J. Am. Chem. Soc. 126, 1465–1477 (2004)
B.D. Alexander, T.J. Dines, Ab initio calculations of the structures and vibrational spectra of ethene complexes. J. Phys. Chem. A 108, 146–156 (2004)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, Gaussian 09, Revision D.01 (Gaussian, Inc., Wallingford CT, 2009)
W. Brands-Williams, M.E. Cuvelier, C.L.W. Berset, Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 18, 25–30 (1995)
T.C.P. Dinis, V.M.C. Madeira, L.M. Almeida, Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 315, 161–169 (1994)
A.A. Osowole, S.M. Wakil, M.O. Emmanuel, Synthesis, characterization, antioxidant and antimicrobial activities of some metal(II) complexes of the mixed-ligands, vitamin B2 and benzoic acid. Elixir Appl. Chem. 79, 30370–30374 (2015)
A.R.H. Pramanik, P.C. Paul, P. Mondal, C.R. Bhattacharjee, Mixed ligand complexes of cobalt(III) and iron(III) containing N2O2-chelating Schiff base: synthesis, characterisation, antimicrobial activity, antioxidant and DFT study. J. Mol. Struct. 1100, 496–505 (2015)
T. Prateek, C. Sulekh, B.S. Saraswat, S. Deepansh, Design, spectral characterization, DFT and biological studies of transition metal complexes of Schiff base derived from 2-aminobenzamide, pyrrole and furan aldehyde. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 143, 1–11 (2015)
D.C. Onwudiwe, A.C. Ekennia, Synthesis, characterisation, thermal, antimicrobial and antioxidant studies of some transition metal dithiocarbamates. Res. Chem. Intermed. (2016). doi:10.1007/s1116-016-2709-2
W.J. Geary, The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 7, 81–122 (1971)
M. Belcastro, T. Marino, N. Russo, M. Toscano, Interaction of cysteine with Cu2+ and group IIb (Zn2+, Cd2+, Hg2+) metal cations: a theoretical study. J. Mass Spectrom. 40, 300–306 (2005)
R.A. Lal, S. Adhikari, A. Kumar, M.L. Pal, Oxoperoxomolybdenum(vi) complexes derived from n-benzamidosalicylaldimine. J. Indian Chem. Soc. 75, 345–349 (1998)
S. Majumder, G.S. Panda, S.K. Choudhuri, Synthesis, characterization and biological properties of a novel copper complex. Eur. J. Med. Chem. 38, 893–898 (2003)
C.S. Marvel, S.A. Aspey, E.A. Dudley, Quadridentate and sexadentate chelates. Some preliminary studies in their preparation and thermal stability. J. Am. Chem. Soc. 78, 4905–4909 (1956)
S.N. Rao, K.N. Munshi, N.N. Rao, M.M. Bhadbhade, E. Suresh, Synthesis, spectral and X-ray structural characterization of [cis-MoO 2 (L)(solv)](L = salicylidene salicyloyl hydrazine) and its use as catalytic oxidant. Polyhedron 18, 2491–2497 (1999)
M.G. Abd El-Wahed, M.S. Refat, S.M. El-Megharbel, Metal complexes of antiuralethic drug: synthesis, spectroscopic characterization and thermal study on allopurinol complexes. J. Mol. Struct. 888, 416–429 (2008)
D. Nicholls, Comprehensive Inorganic Chemistry, vol. 3 (Wiley, Hoboken, 1973)
B. Tang, J.H. Ye, X.H. Ju, Computational study of coordinated Ni(II) complex with high nitrogen content ligands. ISRN Org. Chem. (2011). doi:10.5402/2011/920753
A. Nimmermark, L. Ohrstrom, Z. Reedijk, Metal-ligand bond lengths and strengths: are they correlated? A detailed CSD analysis. Z. Kristallogr. 228, 311–317 (2013)
T.H. Lu, C.S. Chung, T.J. Ashida, Chin. Chem. Soc. 38, 147–154 (1991)
C. Ravikumar, I.H. Joe, V.S. Jayakumar, Charge transfer interactions and nonlinear optical properties of push–pull chromophore benzaldehyde phenylhydrazone: a vibrational approach. Chem. Phys. Lett. 460, 552–558 (2008)
J.V. Burda, J. Sponer, P. Hobza, Ab initio study of the interaction of guanine and adenine with various mono- and bivalent metal cations (Li+, Na+, K+, Rb+, Cs+; Cu+, Ag+, Au+; Mg2+, Ca2+, Sr2+, Ba2+; Zn2+, Cd2+, and Hg2+). J. Phys. Chem. 100, 7250–7255 (1996)
A.C. Ekennia, D.C. Onwudiwe, L.O. Olasunkanmi, A.A. Osowole, E.E. Ebenso, Synthesis, biological and quantum chemical studies of Zn (II) and Ni(II) mixed ligand complexes derived from N,-disubstituted dithiocarbamate and benzoic acid. Bioinorg. Chem. Appl. 2015, 1–12 (2015)
J. Chocholousova, V. Spirko, P. Hobza, First local minimum of the formic acid dimer exhibits simultaneously red-shifted O–H···O and improper blue-shifted C–H···O hydrogen bonds. Phys. Chem. Chem. Phys. 6, 37–41 (2004)
V. Uivarosi, M. Badea, V. Aldea, L. Chirigiu, R. Olar, Thermal and spectral studies of palladium(II) and platinum(IV) complexes with dithiocarbamate derivatives. J. Therm. Anal. Calorim. 111, 1177–1182 (2013)
N. Raman, J.D. Raja, Synthesis, structural characterization and antibacterial studies of some biosensitive mixed ligand copper(II) complexes. Indian J. Chem. 46A, 1611–1614 (2007)
B.D. Corbin, E.H. Seeley, A. Raab, J. Feldmann, M.R. Miller, V.J. Torres, K.L. Anderson, B.M. Dattilo, P.M. Dunman, R. Gerads, R.M. Caprioli, W. Nacken, W.J. Chazin, E.P. Skaar, Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962–965 (2008)
N. Dharmaraj, P. Viswanathamurthi, K. Nataraj, Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity. Transit. Met. Chem. 26, 105–109 (2001)
M.E. De-Leo, A. Tranghee, M. Passantino, A. Mordente, M.M. Lizzio, T. Galeotti, A. Zoli, Manganese superoxide dismutase, glutathione peroxidase, and total radical trapping antioxidant capacity in active rheumatoid arthritis. J. Rheumatol. 29, 2245–2246 (2002)
A. Mahajan, V.R. Tandon, Antioxidants and rheumatoid arthritis. J. Indian Rheumatol. Assoc. 12, 139–142 (2004)
B. Halliwell, J.M.C. Gutteridge, Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219, 1–14 (1984)
A. Guder, H. Korkmaz, Investigation of antioxidant activity and total anthocyanins from blackberry (Rubus hirtus Waldst. and Kit) and cherry laurel (Laurocerasus officinalis Roem.). Asian J. Chem. 24, 4525–4531 (2012)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ekennia, A.C., Osowole, A.A., Olasunkanmi, L.O. et al. Coordination behaviours of new (bidentate N,O-chelating) Schiff bases towards copper(II) and nickel(II) metal ions: synthesis, characterization, antimicrobial, antioxidant, and DFT studies. Res Chem Intermed 43, 3787–3811 (2017). https://doi.org/10.1007/s11164-016-2841-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2841-z