Abstract
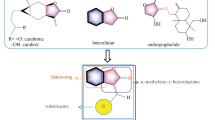
A novel series of fluorinated 1,2,4-triazolo[3,4-b]benzothiazoles was synthesized by fusion of proven antifungal lead 1,2,4-triazole with flourinated benzothiazole nucleus exploiting lead hybridization strategy. Their in vitro antifungal assay against various phytopathogenic fungi revealed that a 2- or 3-chlorinated aryl group at the 3-position of the fused system yielded outstanding and remarkable results of fungitoxicity. Compounds 3b and 3c were found to inflict the best fungitoxicity against most of the test fungi (EC50 value as low as 0.24 mmoles/L) with results better than or comparable to standards. In silico molecular docking and Lipinskii indices were in agreement with the observed trend of antifungal activity. Moreover, the toxicity analysis showed that the compounds belong to class III of toxicity which is the same as that of the recommended standards used.


Similar content being viewed by others
References
A. Sidhu, S. Kukreja, Synthesis of novel fluorinated benzothiazol-2-yl-1,2,4-triazoles: molecular docking, antifungal evaluation and in silico evaluation for SAR. Arab. J. Chem. (2015). doi:10.1016/j.arabjc.2015.01.009
Z. Rezaei, S. Khabnadideh, K. Pakshir, Z. Hossaini, F. Amiri, E. Assadpour, Design, synthesis, and antifungal activity of triazole and benzotriazole derivatives. Eur. J. Med. Chem. 44, 3064–3067 (2009)
Y.S. Wu, H.K. Lee, S.F.Y. Li, High performance chiral separation of fourteen triazole fungicides by sulfated β-cyclodextrin-mediated capillary electrophoresis. J. Chromatogr. 912, 171–179 (2001)
F. Bentiss, M. Lagrenee, M. Traisnel, J.C. Hornez, The corrosion inhibition of mild steel in acidic media by a new triazole derivative. Corros. Sci. 41, 789–803 (1999)
H. Hof, Is there a serious risk of resistance development to azoles among fungi due to the widespread use and long-term application of azole antifungals in medicine? Drug Resist. Updat. 11, 25–31 (2008)
M. Koparir, A. Cansiz, A. Demirdag, Synthesis of some new 4,5-substituted-4H-1,2,4-triazole-3-thiol derivatives. Molecules 9, 204–212 (2004)
P. Naresh, P. Pattanaik, R.L. Priyadarshini, D.R. Reddy, Synthetic characterization & antimicrobial screening of some novel 6-fluorobenzothiazole substituted [1,2,4] triazole analogues. Int. J. Pharm. Res. Health Sci. 1, 18–24 (2013)
X. Chai, J. Zhang, Y. Cao, Y. Zou, Q. Wu, D. Zhang, Y. Jiang, Q. Sun, Design, synthesis and molecular docking studies of novel triazole as antifungal agent. Eur. J. Med. Chem. 46, 3167–3176 (2011)
T. Kumamoto, K. Toyooka, M. Nishida, H. Kuwahara, Y. Yoshimura, J. Kawada, S. Kubota, Effect of 2,4-dihydro-3H-1,2,4-triazole-3-thiones and thiosemicarbazones on iodide uptake by the mouse thyroid: the relationship between their structure and anti-thyroid activity. Chem. Pharm. Bull. 38, 2595–2596 (1990)
I. Scholz, H. Oberwittler, K.D. Riedel, J. Burhenne, J. Weiss, W.E. Haefeli, G. Mikus, Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br. J. Clin. Pharmacol. 68, 906–915 (2009)
H.A. Torres, R.Y. Hachem, R.F. Chemaly, D. Kontoyiannis, I.I. Raad, Posaconazole: a broad-spectrum triazole antifungal. Lancet Infect. Dis. 12, 775–785 (2005)
W. Huang, G.F. Yang, Microwave-assisted, one pot synthesis and fungicidal activity of polyfluorinated 2-benzylthiobenzothiazoles. Bioorg. Med. Chem. 14, 8280–8285 (2006)
K. Gumber, A. Sidhu, V. Kumar, Green synthesis of thiazol-2ylthiazolidin-4-ones as potential antifungals Russian. J. Appl. Chem. 88, 2065–2073 (2015)
T.F. Abbs Fen Rejia, K.N. Rajasekharan, Synthesis of 2-[2,4-diaminothiazol-5-oyl]benzothiazoles. J. Heterocycl. Chem. 47, 994–997 (2010)
C.P. Mpamhanga, D. Spinks, L.B. Tulloch, E.J. Shanks, D.A. Robinson, I.T. Collie, A.H. Fairlamb, P.G. Wyatt, J.A. Frearson, W.N. Hunter, I.H. Gilbert, R. Brenk, One scaffold, three binding modes: novel and selective pteridine reductase 1 inhibitors derived from fragment hits discovered by virtual screening. J. Med. Chem. 52, 4454–4465 (2009)
G. Alang, R. Kaur, G. Kaur, A. Singh, P. Singla, Synthesis and antibacterial activity of some new benzothiazole derivatives. Acta Pharm. Sci. 52, 213–218 (2010)
S.G. Kini, S. Choudhary, M. Mubeen, Synthesis, docking study and anticancer activity of coumarin substituted derivatives of benzothiazole. J. Comput. Methods Mol. Des. 2, 51–60 (2012)
V.N. Telvekar, V.K. Bairwa, K. Satardekar, A. Bellubi, Novel 2-(2-(4-aryloxybenzylidene) hydrazinyl)benzothiazole derivatives as anti-tubercular agents. Bioorg. Med. Chem. Lett. 22, 649–652 (2012)
C. Praveen, A. Nandakumar, P. Dheenkumar, D. Muralidharan, P.T. Perumal, Microwave-assisted one-pot synthesis of benzothiazole and benzoxazole libraries as analgesic agents. J. Chem. Sci. 124, 609–624 (2012)
I. Hutchinson, S.A. Jennings, B.R. Vishnuvajjala, A.D. Westwell, M.F.G. Stevens, Synthesis and pharmaceutical properties of antitumor 2-(4-aminophenyl)benzothiazole amino acid prodrugs. J. Med. Chem. 45, 744–747 (2002)
A. Rana, N. Siddiqui, S.A. Khan, Benzothiazoles: a new profile of biological activities Indian. J. Pharm. Sci. 69, 10–17 (2007)
M. Ban, H. Taguchi, T. Katsushima, M. Takahashi, K. Shinoda, Novel antiallergic and antiinflammatory agents. Part I: synthesis and pharmacology of glycolic amide derivatives. Bioorg. Med. Chem 6, 1069–1076 (1998)
D. Cressier, C. Prouillac, P. Hernandez, C. Amourette, M. Diserbo, C. Lion, G. Rima, Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorg. Med. Chem. 17, 5275–5284 (2009)
J.C. Lee, M.D. Karve, U.S Patent 5,413,795. Chem. Abstr. (1995)
W. Li, Q. Wu, Y. Ye, M. Luo, L. Hu, Y. Gu, F. Niu, J. Hu, Density functional theory and ab initio studies of geometry, electronic structure and vibrational spectra of novel benzothiazole and benzotriazole herbicides. Spectrochim. Acta Mol. Biomol. Spectros. 60, 2343–2354 (2004)
A. Imramovsky, M. Pesko, J. Jampilek, K. Kralova, 1,3-Substituted imidazolidine-2,4,5-triones: synthesis and inhibition of cholinergic enzymes. Monatsh. Chem. 26, 1434–1440 (2014)
T. Fujiwara, D.O. Hagan, Successful fluorine-containing herbicide agrochemicals. J. Fluorine Chem. 167, 16–29 (2014)
L. Li, M. Li, H. Chi, J. Yang, Z. Li, C. Liu, Discovery of flufenoxystrobin: Novel fluorine-containing strobilurin fungicide and acaricide. J. Fluorine Chem. 185, 173–180 (2016)
W. Jian, D. He, P. Xi, X. Li, Synthesis and biological evaluation of novel fluorine-containing stilbene derivatives as fungicidal agents against phytopathogenic fungi. J. Agric. Food Chem. 63(45), 9963–9969 (2015)
M.N. Xanthopoulou, S.K. Hadjikakou, N. Hadjiliadis, M. Kubicki, S. Karkabounas, K. Haralabopoulos, N. Kourkoumelis, T. Bakas, Synthesis and characterization of a new chloro-di-phenyltin(IV) complex with 2-mercapto-nicotinic acid: study of its influence upon the catalytic oxidation of linoleic acid to hydroperoxylinoleic acid by the enzyme lipoxtgenase. J. Organomet. Chem. 691, 1780–1789 (2009)
S.S. Kaki, C. Grey, P. Adlercreutz, Bioorganic synthesis, characterization and antioxidant activity of esters of natural phenolics and α-lipoic acid. J. Biotech. 157(344–349), 40 (2012)
A. Puratchikody, M. Doble, N. Ramalakshmi, Toxicity risk assessment of some novel quinoxaline fused thiazolidinones. J. Pharm. Res. 5, 340–342 (2012)
K.P.S. Adinarayana, P.A. Reddy, P.A. Babu, Structural studies on docking selective COX-2 inhibitors. J. Bioinform. Res. 1, 21–26 (2012)
S. Joginipelli, V.K. Melapu, J. Darsey, Combination of molecular modeling and quantum mechanical studies to understand quinolone resistance mechanism of mycobacterium tuberculosis austin. J. Comput. Biol. Bioinform. 1(2), 5 (2014)
T.R. Devi, G.K.N. Chhetry, Evaluation of antifungal activities of certain plant against Fusarium udum Butler causing wilt in pigeonpea (Cajanus cajan (L.) Millsp.). Int. J. Sci. Res. Pub. 2, 1–4 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kukreja, S., Sidhu, A. & Sharma, V.K. Synthesis of novel 7-fluoro-3-substituted-1,2,4-triazolo[3,4-b]benzothiazoles (FTBs) as potent antifungal agents: molecular docking and in silico evaluation. Res Chem Intermed 42, 8329–8344 (2016). https://doi.org/10.1007/s11164-016-2599-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2599-3