Abstract
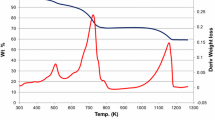
In the preparation of MgO, Mg(OH)2 was placed in a muffle furnace maintained at a desired temperature for rapid increase to the activation temperature and for short-time heating. The MgO obtained showed a higher activity for the retro-aldol reaction of diacetone alcohol and larger surface area in comparison with that prepared by conventional procedures. Among the prepared catalysts, MgO heated at 673 K for 20 min showed the highest activity. The prepared MgO has a wide and thin plate shape with a small crystal size. The surface area of MgO decreased monotonously with increase in heating time and temperature. Further thermal treatment produced a decrease in catalytic activity. After that, the activity was regenerated by a long-time thermal treatment. The change in crystal morphology and exposed surface crystal plane of MgO by thermal treatment is discussed.











Similar content being viewed by others
References
K. Tanabe, M. Misono, Y. Ono, H. Hattori, New Solid Acids and Bases (Kodansha-Elsevier, Tokyo, 1989), p. 29
H. Hattori, Appl. Catal. A 222, 247 (2001)
K.J. Klabunde, H. Matsuhashi, J. Am. Chem. Soc. 109, 1111 (1987)
N. Sutradhar, A. Sinhamahapatra, S.K. Pahari, P. Pal, H.C. Bajaj, I. Mukhopadhyay, A.B. Panda, J. Phys. Chem. C 115, 12308 (2011)
J. Green, J. Mater. Sci. 18, 637 (1983)
H. Onishi, C. Egawa, T. Aruga, Y. Iwasawa, Surf. Sci. 191, 479 (1987)
S. Coluccia, A.J. Tench, Stud. Surf. Sci. Catal. 7, 1160 (1981)
R. Plass, J. Feller, M. Gajdardziska-Josifovska, Surf. Sci. 414, 26 (1998)
M. Chiesa, M.C. Paganini, G. Spoto, E. Giamello, C. Di Valentin, A. Del Vitto, G. Pacchioni, J. Phys. Chem. B 109, 7314 (2005)
P.S. Das, D.K.R. Chanda, A. Dey, P.S. Das, A.K. Mandal, K.D. Gupta, N. Dey, A.K. Mukhopadhyay, D. Kr, Ceram. Int. 40, 6365 (2014)
Acknowledgments
Authors acknowledge Mr. D. Inoue for his assistance with the SEM images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitagawa, M., Misu, S., Ichikawa, J. et al. Preparation of active MgO by short-time thermal decomposition of Mg(OH)2 . Res Chem Intermed 41, 9463–9473 (2015). https://doi.org/10.1007/s11164-015-1971-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-1971-z