Abstract
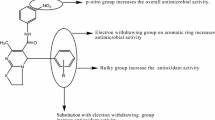
Two new series of methyl 7-methyl-5-(substituted-phenyl)-3,5-dihydro-2H—substituted [3,2-α]pyrimidine-6-carboxylate derivatives of thiourea and guanidine were synthesized. These compounds were characterized and evaluated for their antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and antifungal Aspergillus niger and Candida albicans and free radical scavenging activity using DPPH reagent method. Compound 7f was found to be the most active antibacterial and antifungal agent comparable to the standard drugs ciprofloxacin and fluconazole. Further, the compounds 5e, 7g, and 7h were also found to have significant antimicrobial activity. Compound 5a was found to be the most active antioxidant among all the targeted compounds, while compounds 5b, 5g, 7b, and 7f also had significant antioxidant activity compared to standard ascorbic acid.


Similar content being viewed by others
References
I.C. Zampini, S. Cuello, M.R. Alberto, R.M. Ordonez, R.D. Almeida, E. Solorzano, M.I. Isla, J. Ethnopharmacol. 124, 499–505 (2009)
H. Mitsuya, R. Yarchoan, S. Broder, Science 249, 1533–1544 (1990)
E. Celep, A. Aydın, H. Kırmızıbekmez, E. Yesilada, Food Chem. Toxicol. 62, 448–455 (2013)
T. Finkel, Mol. Cell Biol. 6, 971–976 (2005)
T. Finkel, N.J. Holbrook, Oxidants. Nature 408, 239–247 (2000)
P. Biginelli, Gazz. Chim. Ital. 23, 360 (1893)
K.S. Kumar, A.V. Kanth, K. Tatendra Reddy, G. Omprakash, J. Chem. Pharm. Res. 3(5), 234–252 (2011)
T. Gazivoda, M. Plevnik, J. Plavec, S. Kraljevic, M. Kralj, K. Pavelic, Bioorg. Med. Chem. 13, 131–139 (2005)
K.M. Hilmy, M.M. Khalifa, M.A. Hawata, R.M. Keshk, A.A. Torgman, Eur. J. Med. Chem. 45, 5243–5250 (2010)
V.P. Krivonogov, V.A. Myshkin, G.A. Sivkova, N.A. Greben’kova, D.V. Srubillin, G.G. Kozlova, I.B. Abdrakhmanov, R.T. Mannapova, L.V. Spirikhin, G.A. Tolstiko, Pharm. Chem. J. 35, 8–10 (2001)
H.Z. Hafez, A.B. El-Gazzar, Bioorg. Med. Chem. Lett. 18, 5222–5227 (2008)
M. Ban, H. Taguchi, T. Katsushima, T. Aoki, Bioorg. Med. Chem. 6, 1057–1067 (1998)
A.S. Elgazwy, N.S. Ismail, H.S. Elzahabi, Bioorg. Med. Chem. 18, 7639–7650 (2010)
B. Sati, H. Sati, L.V. Nargund, S. Khaidem, C.P. Bhatt, S. Saklani, Orient. J. Chem. 28, 1055–1059 (2012)
R. Kumar, A. Mittal, U. Ramachandran, Bioorg. Med. Chem. Lett. 17, 4613–4618 (2007)
S. Sigroha, B. Narasimhan, P. Kumar, A. Khatkar, K. Ramasamy, V. Mani, R.K. Mishra, A.A. Majeed, Med. Chem. Res. 21, 3863–3875 (2011)
A. Khatkar, A. Nanda, P. Kumar, B. Narasimhan, Res. Chem. Intermed. (2013a). doi:10.1007/s11164-013-1192-1202
A. Khatkar, A. Nanda, P. Kumar, B. Narasimhan, Arab. J. Chem. (2013b). doi:10.1016/j.arabjc.2013.11.014
D. Akbari, P.K. Kachhadia, S.D. Tala, A.H. Bapodra, M.F. Dhaduk, H.S. Joshi, K.B. Mehta, S.J. Pathak, Phosphorus Sulfur Silicon Relat. Elem. 183, 1911–1922 (2008)
J.G. Cappucino, N. Sherman, Microbiology-a laboratory manual, 2nd edn. (Addison Wesley, California, 1999), p. 263
K. Sancak, Y. Unver, D. Unluer, E. Dugdu, G. Kor, F. Celik, Turk. J. Chem. 36, 457–466 (2012)
P. Sharma, N. Rane, V.K. Gurram, Bioorg. Med. Chem. Lett. 14, 4185–4190 (2004)
V. Bondet, W. Brand-Williams, C. Berset, Food Sci Technol 30, 609–615 (1997)
C.M. Bhalgat, M.I. Ali, B. Ramesh, G. Ramu, Arabian. J. Chem. (2011), doi:10.1016/j.arabjc.2010.12.021
P. Mondal, S. Jana, L.K. Kanthal et al., T. Ph. Res. 3, 17–26 (2010)
S.M. Kumar, M. Pavani, C.M. Bhalgat, R. Deepthi, A. Mounika, S.R. Mudshinge, Int. J. Res. Pharm. Biomed. Sci. 2, 1568–1570 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malik, N., Dhiman, P., Verma, P.K. et al. Design, synthesis, and biological evaluation of thiourea and guanidine derivatives of pyrimidine-6-carboxylate. Res Chem Intermed 41, 7981–7993 (2015). https://doi.org/10.1007/s11164-014-1871-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1871-7