Abstract
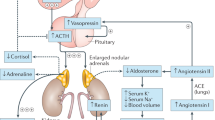
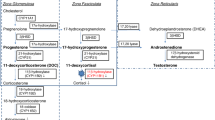
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency (21-OHD) is an autosomal-recessive disease causing cortisol deficiency, aldosterone deficiency and hyperandrogenism. Diagnosis of 21-OHD is confirmed by steroid analysis in newborn screening or later on. Standard medical treatment consists of oral glucocorticoid and mineralocorticoid administration in order to suppress adrenal androgens and to compensate for adrenal steroid deficiencies. However, available treatment is far from ideal, and not much is known about the long-term outcome in CAH as trials in patients in adulthood or old age are rare. Here we briefly describe the pathophysiology, clinical picture, genetics and epidemiology of 21-OHD. This is followed by a comprehensive review of the recent advances in diagnosis, treatment and outcome. Novel insights have been gained in the fields of newborn screening, specific steroid measurement utilizing mass spectrometry, genetics, glucocorticoid stress dosing, additive medical therapy, prenatal treatment, side-effects of medical treatment, adrenomedullary involvement, metabolic morbidity, fertility and gender identity. However, many issues are still unresolved, and novel questions, which will have to be answered in the future, arise with every new finding.
Similar content being viewed by others
References
Wilkins L, Lewis RA, Klein R, Rosemberg E. The suppression of androgen secretion by cortisone in a case of congenital adrenal hyperplasia. Bull Johns Hopkins Hosp 1950;86:249–52.
Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med 2003;349:776–88.
Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet 2005;365:2125–36.
Krone N, Dhir V, Ivison HE, Arlt W. Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin Endocrinol (Oxf) 2007;66:162–72.
Merke DP, Chrousos GP, Eisenhofer G, Weise M, Keil MF, Rogol AD, et al. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med 2000;343:1362–8.
Prader A, Gurtner HP. The syndrome of male pseudohermaphrodism in congenital adrenocortical hyperplasia without overproduction of androgens (adrenal male pseudohermaphrodism). Helv Paediatr Acta 1955;10:397–412.
Pang S, Shook MK. Current status of neonatal screening for congenital adrenal hyperplasia. Curr Opin Pediatr 1997;9:419–23.
Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab 2000;85:1059–65.
Levine LS, Zachmann M, New MI, Prader A, Pollack MS, O’Neill GJ, et al. Genetic mapping of the 21-hydroxylase-deficiency gene within the HLA linkage group. N Engl J Med 1978;299:911–5.
White PC, Tusie-Luna MT, New MI, Speiser PW. Mutations in steroid 21-hydroxylase (CYP21). Hum Mutat 1994;3:373–8.
White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev 2000;21:245–91.
Jaaskelainen J, Levo A, Voutilainen R, Partanen J. Population-wide evaluation of disease manifestation in relation to molecular genotype in steroid 21-hydroxylase (CYP21) deficiency: good correlation in a well defined population. J Clin Endocrinol Metab 1997;82:3293–7.
Speiser PW, Dupont J, Zhu D, Serrat J, Buegeleisen M, Tusie-Luna MT, et al. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest 1992;90:584–95.
Riepe FG, Tatzel S, Sippell WG, Pleiss J, Krone N. Congenital adrenal hyperplasia: the molecular basis of 21-hydroxylase deficiency in H-2(aw18) mice. Endocrinology 2005;146:2563–74.
Brosnan PG, Brosnan CA, Kemp SF, Domek DB, Jelley DH, Blackett PR, et al. Effect of newborn screening for congenital adrenal hyperplasia. Arch Pediatr Adolesc Med 1999;153:1272–8.
Van der Kamp HJ, Noordam K, Elvers B, Van Baarle M, Otten BJ, Verkerk PH. Newborn screening for congenital adrenal hyperplasia in the Netherlands. Pediatrics 2001;108:1320–4.
Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet 1985;37:650–67.
Honour JW, Torresani T. Evaluation of neonatal screening for congenital adrenal hyperplasia. Horm Res 2001;55:206–11.
Loeber JG. Neonatal screening in Europe; the situation in 2004. J Inherit Metab Dis 2007.
Balsamo A, Cacciari E, Piazzi S, Cassio A, Bozza D, Pirazzoli P, et al. Congenital adrenal hyperplasia: neonatal mass screening compared with clinical diagnosis only in the Emilia-Romagna region of Italy, 1980–1995. Pediatrics 1996;98:362–7.
Therrell BL, Jr, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, et al. Results of screening 1.9 million Texas newborns for 21hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics 1998;101:583–90.
Steigert M, Schoenle EJ, Biason-Lauber A, Torresani T. High reliability of neonatal screening for congenital adrenal hyperplasia in Switzerland. J Clin Endocrinol Metab 2002;87:4106–10.
Sippell WG, Bidlingmaier F, Becker H, Brunig T, Dorr H, Hahn H, et al. Simultaneous radioimmunoassay of plasma aldosterone, corticosterone, 11-deoxycorticosterone, progesterone, 17-hydroxyprogesterone, 11-deoxycortisol, cortisol and cortisone. J Steroid Biochem 1978;9:63–74.
Wudy SA, Wachter UA, Homoki J, Teller WM. 17 alpha-hydroxyprogesterone, 4androstenedione, and testosterone profiled by routine stable isotope dilution/gas chromatography-mass spectrometry in plasma of children. Pediatr Res 1995;38:76–80.
Wudy SA, Hartmann M, Svoboda M. Determination of 17-hydroxyprogesterone in plasma by stable isotope dilution/benchtop liquid chromatography-tandem mass spectrometry. Horm Res 2000;53:68–71.
Lai CC, Tsai CH, Tsai FJ, Wu JY, Lin WD, Lee CC. Rapid screening assay of congenital adrenal hyperplasia by measuring 17 alpha-hydroxyprogesterone with high-performance liquid chromatography/electrospray ionization tandem mass spectrometry from dried blood spots. J Clin Lab Anal 2002;16:20–5.
Kao PC, Machacek DA, Magera MJ, Lacey JM, Rinaldo P. Diagnosis of adrenal cortical dysfunction by liquid chromatography-tandem mass spectrometry. Ann Clin Lab Sci 2001;31:199–204.
Caulfield MP, Lynn T, Gottschalk ME, Jones KL, Taylor NF, Malunowicz EM, et al. The diagnosis of congenital adrenal hyperplasia in the newborn by gas chromatography/mass spectrometry analysis of random urine specimens. J Clin Endocrinol Metab 2002;87:3682–90.
Lacey JM, Minutti CZ, Magera MJ, Tauscher AL, Casetta B, McCann M, et al. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clin Chem 2004;50:621–5.
Minutti CZ, Lacey JM, Magera MJ, Hahn SH, McCann M, Schulze A, et al. Steroid profiling by tandem mass spectrometry improves the positive predictive value of newborn screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab 2004;89:3687–93.
Janzen N, Peter M, Sander S, Steuerwald U, Terhardt M, Holtkamp U, et al. Newborn screening for congenital adrenal hyperplasia: additional steroid profile using liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab 2007;92:2581–9.
Knorr D, Albert ED, Bidlingmaier F, Holler W, Scholz S. Different gene defects in the salt-wasting (SW), simple virilizing (SV), and nonclassical (NC) types of congenital adrenal hyperplasia (CAH). Ann N Y Acad Sci 1985;458:71–5.
White PC, Grossberger D, Onufer BJ, Chaplin DD, New MI, Dupont B, et al. Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man. Proc Natl Acad Sci USA 1985;82:1089–93.
White PC, Vitek A, Dupont B, New MI. Characterization of frequent deletions causing steroid 21-hydroxylase deficiency. Proc Natl Acad Sci USA 1988;85:4436–40.
Morel Y, Andre J, Uring-Lambert B, Hauptmann G, Betuel H, Tossi M, et al. Rearrangements and point mutations of P450c21 genes are distinguished by five restriction endonuclease haplotypes identified by a new probing strategy in 57 families with congenital adrenal hyperplasia. J Clin Invest 1989;83:527–36.
Olney RC, Mougey EB, Wang J, Shulman DI, Sylvester JE. Using real-time, quantitative PCR for rapid genotyping of the steroid 21-hydroxylase gene in a north Florida population. J Clin Endocrinol Metab 2002;87:735–41.
Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 2002;30:e57.
Higashi Y, Tanae A, Inoue H, Fujii-Kuriyama Y. Evidence for frequent gene conversion in the steroid 21-hydroxylase P-450(C21) gene: implications for steroid 21-hydroxylase deficiency. Am J Hum Genet 1988;42:17–25.
Wedell A, Ritzen EM, Haglund-Stengler B, Luthman H. Steroid 21-hydroxylase deficiency: three additional mutated alleles and establishment of phenotype-genotype relationships of common mutations. Proc Natl Acad Sci USA 1992;89:7232–6.
Tajima T, Fujieda K, Nakayama K, Fujii-Kuriyama Y. Molecular analysis of patient and carrier genes with congenital steroid 21-hydroxylase deficiency by using polymerase chain reaction and single strand conformation polymorphism. J Clin Invest 1993;92:2182–90.
Day DJ, Speiser PW, White PC, Barany F. Detection of steroid 21-hydroxylase alleles using gene-specific PCR and a multiplexed ligation detection reaction. Genomics 1995;29:152–62.
Lee HH, Chao HT, Ng HT, Choo KB. Direct molecular diagnosis of CYP21 mutations in congenital adrenal hyperplasia. J Med Genet 1996;33:371–5.
Krone N, Braun A, Weinert S, Peter M, Roscher AA, Partsch CJ, et al. Multiplex minisequencing of the 21-hydroxylase gene as a rapid strategy to confirm congenital adrenal hyperplasia. Clin Chem 2002;48:818–25.
Kosel S, Burggraf S, Fingerhut R, Dorr HG, Roscher AA, Olgemoller B. Rapid second-tier molecular genetic analysis for congenital adrenal hyperplasia attributable to steroid 21-hydroxylase deficiency. Clin Chem 2005;51:298–304.
Keen-Kim D, Redman JB, Alanes RU, Eachus MM, Wilson RC, New MI, et al. Validation and clinical application of a locus-specific polymerase chain reaction- and minisequencing-based assay for congenital adrenal hyperplasia (21-hydroxylase deficiency). J Mol Diagnostics 2005;7:236–46.
Higashi Y, Hiromasa T, Tanae A, Miki T, Nakura J, Kondo T, et al. Effects of individual mutations in the P-450(C21) pseudogene on the P-450(C21) activity and their distribution in the patient genomes of congenital steroid 21-hydroxylase deficiency. J Biochem (Tokyo) 1991;109:638–44.
Wedell A, Thilen A, Ritzen EM, Stengler B, Luthman H. Mutational spectrum of the steroid 21-hydroxylase gene in Sweden: implications for genetic diagnosis and association with disease manifestation. J Clin Endocrinol Metab 1994;78:1145–52.
Tukel T, Uyguner O, Wei JQ, Yuksel-Apak M, Saka N, Song DX, et al. A novel semiquantitative polymerase chain reaction/enzyme digestion-based method for detection of large scale deletions/conversions of the CYP21 gene and mutation screening in Turkish families with 21-hydroxylase deficiency. J Clin Endocrinol Metab 2003;88:5893–7.
White PC, New MI, Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci USA 1984;81:7505–9.
Tusie-Luna MT, Traktman P, White PC. Determination of functional effects of mutations in the steroid 21-hydroxylase gene (CYP21) using recombinant vaccinia virus. J Biol Chem 1990;265:20916–22.
Tusie-Luna MT, Speiser PW, Dumic M, New MI, White PC. A mutation (Pro-30 to Leu) in CYP21 represents a potential nonclassic steroid 21-hydroxylase deficiency allele. Mol Endocrinol 1991;5:685–92.
Helmberg A, Tusie-Luna MT, Tabarelli M, Kofler R, White PC. R339H and P453S: CYP21 mutations associated with nonclassic steroid 21-hydroxylase deficiency that are not apparent gene conversions. Mol Endocrinol 1992;6:1318–22.
Wilson RC, Mercado AB, Cheng KC, New MI. Steroid 21-hydroxylase deficiency: genotype may not predict phenotype. J Clin Endocrinol Metab 1995;80:2322–9.
Nimkarn S, Lin-Su K, Berglind N, Wilson RC, New MI. Aldosterone-to-renin ratio as a marker for disease severity in 21-hydroxylase deficiency congenital adrenal hyperplasia. J Clin Endocrinol Metab 2007;92:137–42.
Bornstein SR, Tajima T, Eisenhofer G, Haidan A, Aguilera G. Adrenomedullary function is severely impaired in 21-hydroxylase-deficient mice. FASEB J 1999;13:1185–94.
Charmandari E, Eisenhofer G, Mehlinger SL, Carlson A, Wesley R, Keil MF, et al. Adrenomedullary function may predict phenotype and genotype in classic 21-hydroxylase deficiency. J Clin Endocrinol Metab 2002;87:3031–7.
Johannsson G, Bergthorsdottir R, Nilsson A, Lennernas H, Hedner T, Skrtic S. Improved Glucocorticoid Replacement Therapy by a Novel Oral Hydrocortisone Modified-Release Tablet. In: 89th Annual Meeting of the Endocrine Society 2007. Toronto; 2007. p. 4–12.
Clayton PE, Miller WL, Oberfield SE, Ritzen EM, Sippell WG, Speiser PW. Consensus statement on 21-hydroxylase deficiency from the European Society for Paediatric Endocrinology and the Lawson Wilkins Pediatric Endocrine Society. Horm Res 2002;58:188–95.
Riepe FG, Krone N, Viemann M, Partsch CJ, Sippell WG. Management of congenital adrenal hyperplasia: results of the ESPE questionnaire. Horm Res 2002;58:196–205.
Charmandari E, Matthews DR, Johnston A, Brook CG, Hindmarsh PC. Serum cortisol and 17-hydroxyprogesterone interrelation in classic 21-hydroxylase deficiency: is current replacement therapy satisfactory? J Clin Endocrinol Metab 2001;86:4679–85.
Charmandari E, Hindmarsh PC, Johnston A, Brook CG. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency: alterations in cortisol pharmacokinetics at puberty. J Clin Endocrinol Metab 2001;86:2701–8.
Krieg RJ Jr, Santos F, Chan JC. Growth hormone, insulin-like growth factor and the kidney. Kidney Int 1995;48:321–36.
Moore JS, Monson JP, Kaltsas G, Putignano P, Wood PJ, Sheppard MC, et al. Modulation of 11beta-hydroxysteroid dehydrogenase isozymes by growth hormone and insulin-like growth factor: in vivo and in vitro studies. J Clin Endocrinol Metab 1999;84:4172–7.
Balducci R, Toscano V, Larizza D, Mangiantini A, Galasso C, Municchi G, et al. Effects of long-term growth hormone therapy on adrenal steroidogenesis in Turner syndrome. Horm Res 1998;49:210–5.
Rivkees SA, Crawford JD. Dexamethasone treatment of virilizing congenital adrenal hyperplasia: the ability to achieve normal growth. Pediatrics 2000;106:767–73.
Punthakee Z, Legault L, Polychronakos C. Prednisolone in the treatment of adrenal insufficiency: a re-evaluation of relative potency. J Pediatr 2003;143:402–5.
Jansen M, Wit JM, van den Brande JL. Reinstitution of mineralocorticoid therapy in congenital adrenal hyperplasia. Effects on control and growth. Acta Paediatr Scand 1981;70:229–33.
Charmandari E, Lichtarowicz-Krynska EJ, Hindmarsh PC, Johnston A, Aynsley-Green A, Brook CG. Congenital adrenal hyperplasia: management during critical illness. Arch Dis Child 2001;85:26–8.
Charmandari E, Johnston A, Brook CG, Hindmarsh PC. Bioavailability of oral hydrocortisone in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Endocrinol 2001;169:65–70.
Cryer PE. Adrenaline: a physiological metabolic regulatory hormone in humans? Int J Obes Relat Metab Disord 1993;17 Suppl 3:S43–6;discussion S68.
Weise M, Mehlinger SL, Drinkard B, Rawson E, Charmandari E, Hiroi M, et al. Patients with classic congenital adrenal hyperplasia have decreased epinephrine reserve and defective glucose elevation in response to high-intensity exercise. J Clin Endocrinol Metab 2004;89:591–7.
Howlett K, Galbo H, Lorentsen J, Bergeron R, Zimmerman-Belsing T, Bulow J, et al. Effect of adrenaline on glucose kinetics during exercise in adrenalectomised humans. J Physiol 1999;519 Pt 3:911–21.
Hinde FR, Johnston DI. Hypoglycaemia during illness in children with congenital adrenal hyperplasia. Br Med J (Clin Res Ed) 1984;289:1603–4.
McNinch AW, Savage DC. Hypoglycemia during illness in children with congenital adrenal hyperplasia. Br Med J (Clin Res Ed) 1985;290:243.
Artavia-Loria E, Chaussain JL, Bougneres PF, Job JC. Frequency of hypoglycemia in children with adrenal insufficiency. Acta Endocrinol Suppl (Copenh) 1986;279:275–8.
Weise M, Drinkard B, Mehlinger SL, Holzer SM, Eisenhofer G, Charmandari E, et al. Stress dose of hydrocortisone is not beneficial in patients with classic congenital adrenal hyperplasia undergoing short-term, high-intensity exercise. J Clin Endocrinol Metab 2004;89:3679–84.
Coker RH, Kjaer M. Glucoregulation during exercise : the role of the neuroendocrine system. Sports Med 2005;35:575–83.
Green-Golan L, Yates C, Drinkard B, Vanryzin C, Eisenhofer G, Weise M, et al. Patients with classic congenital adrenal hyperplasia have decreased epinephrine reserve and defective glycemic control during prolonged moderate-intensity exercise. J Clin Endocrinol Metab 2007:89:591–7.
Zuckerman-Levin N, Tiosano D, Eisenhofer G, Bornstein S, Hochberg Z. The importance of adrenocortical glucocorticoids for adrenomedullary and physiological response to stress: a study in isolated glucocorticoid deficiency. J Clin Endocrinol Metab 2001;86:5920–4.
Riepe FG, Krone N, Kruger SN, Sweep FC, Lenders JW, Dotsch J, et al. Absence of exercise-induced leptin suppression associated with insufficient epinephrine reserve in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Exp Clin Endocrinol Diabetes 2006;114:105–10.
Laue L, Merke DP, Jones JV, Barnes KM, Hill S, Cutler GB, Jr A preliminary study of flutamide, testolactone, and reduced hydrocortisone dose in the treatment of congenital adrenal hyperplasia. J Clin Endocrinol Metab 1996;81:3535–9.
Merke DP, Keil MF, Jones JV, Fields J, Hill S, Cutler GB, Jr Flutamide, testolactone, and reduced hydrocortisone dose maintain normal growth velocity and bone maturation despite elevated androgen levels in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2000;85:1114–20.
Allen DB. Growth suppression by glucocorticoid therapy. Endocrinol Metab Clin N Am 1996;25:699–717.
Allen DB, Julius JR, Breen TJ, Attie KM. Treatment of glucocorticoid-induced growth suppression with growth hormone. National cooperative growth study. J Clin Endocrinol Metab 1998;83:2824–9.
Pucarelli I, Segni M, Ortore M, Arcadi E, Pasquino AM. Effects of combined gonadotropin-releasing hormone agonist and growth hormone therapy on adult height in precocious puberty: a further contribution. J Pediatr Endocrinol Metab 2003;16:1005–10.
Lin-Su K, Vogiatzi MG, Marshall I, Harbison MD, Macapagal MC, Betensky B, et al. Treatment with growth hormone and luteinizing hormone releasing hormone analog improves final adult height in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2005;90:3318–25.
Merke DP, Bornstein SR, Avila NA, Chrousos GP. NIH conference. Future directions in the study and management of congenital adrenal hyperplasia due to 21hydroxylase deficiency. Ann Intern Med 2002;136:320–34.
Grosse SD, Van Vliet G. How many deaths can be prevented by newborn screening for congenital adrenal hyperplasia? Horm Res 2007;67:284–291.
Yoo BK, Grosse SD. Cost-effectiveness of newborn screening for congenital adrenal hyperplasia: a preliminary analysis. In: National Newborn Screening and Genetic Testing Symposium: 2005. Portland;2005.
Thilen A, Larsson A. Congenital adrenal hyperplasia in Sweden 1969–1986. Prevalence, symptoms and age at diagnosis. Acta Paediatr Scand 1990;79:168–75.
Thilen A, Nordenstrom A, Hagenfeldt L, von Dobeln U, Guthenberg D, Larsson A. Benefits of neonatal screening for congenital adrenal hyperplasia (21-hydroxylase deficiency) in Sweden. Pediatrics 1998;101:E11.
Kovacs J, Votava F, Heinze G, Solyom J, Lebl J, Pribilincova Z, et al. Lessons from 30 years of clinical diagnosis and treatment of congenital adrenal hyperplasia in five middle European countries. J Clin Endocrinol Metab 2001;86:2958–64.
David M, Forest MG. Prenatal treatment of congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency. J Pediatr 1984;105:799–803.
Avent ND, Chitty LS. Non-invasive diagnosis of fetal sex; utilisation of free fetal DNA in maternal plasma and ultrasound. Prenat Diagn 2006;26:598–603.
Seckl JR, Miller WL. How safe is long-term prenatal glucocorticoid treatment? JAMA 1997;277:1077–9.
Raff H. Neonatal dexamethasone therapy: short- and long-term consequences. Trends Endocrinol Metab 2004;15:351–2.
Forest MG, David M, Morel Y. Prenatal diagnosis and treatment of 21-hydroxylase deficiency. J Steroid Biochem Mol Biol 1993;45:75–82.
Lajic S, Wedell A, Bui TH, Ritzen EM, Holst M. Long-term somatic follow-up of prenatally treated children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 1998;83:3872–80.
New MI, Carlson A, Obeid J, Marshall I, Cabrera MS, Goseco A, et al. Prenatal diagnosis for congenital adrenal hyperplasia in 532 pregnancies. J Clin Endocrinol Metab 2001;86:5651–7.
Meyer-Bahlburg HF, Dolezal C, Baker SW, Carlson AD, Obeid JS, New MI. Cognitive and motor development of children with and without congenital adrenal hyperplasia after early-prenatal dexamethasone. J Clin Endocrinol Metab 2004;89:610–4.
Hirvikoski T, Nordenstrom A, Lindholm T, Lindblad F, Ritzen EM, Wedell A, et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab 2007;92:542–8.
Eugster EA, Dimeglio LA, Wright JC, Freidenberg GR, Seshadri R, Pescovitz OH. Height outcome in congenital adrenal hyperplasia caused by 21-hydroxylase deficiency: a meta-analysis. J Pediatr 2001;138:26–32.
Frisch H, Waldhauser F, Lebl J, Solyom J, Hargitai G, Kovacs J, et al. Congenital adrenal hyperplasia: lessons from a multinational study. Horm Res 2002;57 Suppl 2:95–101.
Van der Kamp HJ, Otten BJ, Buitenweg N, De Muinck Keizer-Schrama SM, Oostdijk W, Jansen M, et al. Longitudinal analysis of growth and puberty in 21-hydroxylase deficiency patients. Arch Dis Child 2002;87:139–44.
Balsamo A, Cicognani A, Baldazzi L, Barbaro M, Baronio F, Gennari M, et al. CYP21 genotype, adult height, and pubertal development in 55 patients treated for 21-hydroxylase deficiency. J Clin Endocrinol Metab 2003;88:5680–8.
Pinto G, Tardy V, Trivin C, Thalassinos C, Lortat-Jacob S, Nihoul-Fekete C, et al. Follow-up of 68 children with congenital adrenal hyperplasia due to 21hydroxylase deficiency: relevance of genotype for management. J Clin Endocrinol Metab 2003;88:2624–33.
Bonfig W, Bechtold S, Schmidt H, Knorr D, Schwarz HP. Reduced final height outcome in congenital adrenal hyperplasia under prednisone treatment: deceleration of growth velocity during puberty. J Clin Endocrinol Metab 2007;92:1635–9.
Cornean RE, Hindmarsh PC, Brook CG. Obesity in 21-hydroxylase deficient patients. Arch Dis Child 1998;78:261–3.
Volkl TM, Simm D, Beier C, Dorr HG. Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics 2006;117:e98–105.
Bachelot A, Plu-Bureau G, Thibaud E, Laborde K, Pinto G, Samara D, et al. Long-term outcome of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res 2007;67:268–76.
Stikkelbroeck NM, Oyen WJ, van der Wilt GJ, Hermus AR, Otten BJ. Normal bone mineral density and lean body mass, but increased fat mass, in young adult patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2003;88:1036–42.
Charmandari E, Weise M, Bornstein SR, Eisenhofer G, Keil MF, Chrousos GP, et al. Children with classic congenital adrenal hyperplasia have elevated serum leptin concentrations and insulin resistance: potential clinical implications. J Clin Endocrinol Metab 2002;87:2114–20.
Hague WM, Adams J, Rodda C, Brook CG, de Bruyn R, Grant DB, et al. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf) 1990;33:501–10.
Saygili F, Oge A, Yilmaz C. Hyperinsulinemia and insulin insensitivity in women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency: the relationship between serum leptin levels and chronic hyperinsulinemia. Horm Res 2005;63:270–4.
Roche EF, Charmandari E, Dattani MT, Hindmarsh PC. Blood pressure in children and adolescents with congenital adrenal hyperplasia (21-hydroxylase deficiency): a preliminary report. Clin Endocrinol (Oxf) 2003;58:589–96.
Volkl TM, Simm D, Dotsch J, Rascher W, Dorr HG. Altered 24-hour blood pressure profiles in children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 2006;91:4888–95.
Day C. Metabolic syndrome, or What you will: definitions and epidemiology. Diab Vasc Dis Res 2007;4:32–8.
O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999;340:14–22.
Sartorato P, Zulian E, Benedini S, Mariniello B, Schiavi F, Bilora F, et al. Cardiovascular risk factors and ultrasound evaluation of intima-media thickness at common carotids, carotid bulbs, and femoral and abdominal aorta arteries in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 2007;92:1015–8.
Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjold A, Hagenfeldt K, et al. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 2007;92:110–6.
Mulaikal RM, Migeon CJ, Rock JA. Fertility rates in female patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med 1987;316:178–82.
Krone N, Wachter I, Stefanidou M, Roscher AA, Schwarz HP. Mothers with congenital adrenal hyperplasia and their children: outcome of pregnancy, birth and childhood. Clin Endocrinol (Oxf) 2001;55:523–9.
Stikkelbroeck NM, Hermus AR, Braat DD, Otten BJ. Fertility in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Obstet Gynecol Surv 2003;58:275–84.
Feldman S, Billaud L, Thalabard JC, Raux-Demay MC, Mowszowicz I, Kuttenn F, et al. Fertility in women with late-onset adrenal hyperplasia due to 21hydroxylase deficiency. J Clin Endocrinol Metab 1992;74:635–9.
Moran C, Azziz R, Weintrob N, Witchel SF, Rohmer V, Dewailly D, et al. Reproductive outcome of women with 21hydroxylase-deficient nonclassic adrenal hyperplasia. J Clin Endocrinol Metab 2006;91:3451–6.
Gastaud F, Bouvattier C, Duranteau L, Brauner R, Thibaud E, Kutten F, et al. Impaired sexual and reproductive outcomes in women with classical forms of congenital adrenal hyperplasia. J Clin Endocrinol Metab 2007;92:1391–6.
Crouch NS, Minto CL, Laio LM, Woodhouse CR, Creighton SM. Genital sensation after feminizing genitoplasty for congenital adrenal hyperplasia: a pilot study. BJU Int 2004;93:135–8.
Jaaskelainen J, Hippelainen M, Kiekara O, Voutilainen R. Child rate, pregnancy outcome and ovarian function in females with classical 21-hydroxylase deficiency. Acta Obstet Gynecol Scand 2000;79:687–92.
Stikkelbroeck NM, Otten BJ, Pasic A, Jager GJ, Sweep CG, Noordam K, et al. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2001;86:5721–8.
Cabrera MS, Vogiatzi MG, New MI. Long term outcome in adult males with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab 2001;86:3070–8.
Val P, Jeays-Ward K, Swain A. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev Biol 2006;299:250–6.
Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Span PN, Ross HA, Meuleman EJ, et al. Testicular tumours in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. J Clin Endocrinol Metab 2007.
Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Hermus AR. Repeated successful induction of fertility after replacing hydrocortisone with dexamethasone in a patient with congenital adrenal hyperplasia and testicular adrenal rest tumors. Fertil Steril 2007.
Walker BR, Skoog SJ, Winslow BH, Canning DA, Tank ES. Testis sparing surgery for steroid unresponsive testicular tumors of the adrenogenital syndrome. J Urol 1997;157:1460–3.
Claahsen-van der Grinten HL, Otten BJ, Takahashi S, Meuleman EJ, Hulsbergen-van de Kaa C, Sweep FC, et al. Testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia: evaluation of pituitary-gonadal function before and after successful testis-sparing surgery in eight patients. J Clin Endocrinol Metab 2007;92:612–5.
Ehrhardt AA, Epstein R, Money J. Fetal androgens and female gender identity in the early-treated adrenogenital syndrome. Johns Hopkins Med J 1968;122:160–7.
Collaer ML, Hines M. Human behavioral sex differences: a role for gonadal hormones during early development? Psychol Bull 1995;118:55–107.
Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev 2005;29:353–84.
Zucker KJ. Measurement of psychosexual differentiation. Arch Sex Behav 2005;34:375–88.
Dessens AB, Slijper FM, Drop SL. Gender dysphoria and gender change in chromosomal females with congenital adrenal hyperplasia. Arch Sex Behav 2005;34:389–97.
Meyer-Bahlburg HF, Dolezal C, Baker SW, Carlson AD, Obeid JS, New MI. Prenatal androgenization affects gender-related behavior but not gender identity in 5–12-yearold girls with congenital adrenal hyperplasia. Arch Sex Behav 2004;33:97–104.
Berenbaum SA, Bailey JM. Effects on gender identity of prenatal androgens and genital appearance: evidence from girls with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2003;88:1102–6.
Crecchio D. Sopra un caso di apparenzi virili in una donna. Morgagni 1865;7:154–88.
Acknowledgements
The authors acknowledge an ongoing institutional grant from the Medical Faculty, Christian-Albrechts-Universität zu Kiel and thank Mrs. Joanna Voerste for linguistic assistance with the text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riepe, F.G., Sippell, W.G. Recent advances in diagnosis, treatment, and outcome of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Rev Endocr Metab Disord 8, 349–363 (2007). https://doi.org/10.1007/s11154-007-9053-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-007-9053-1