Abstract
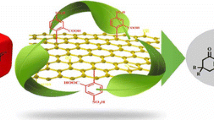
Graphene oxide nanosheets were synthesized from natural graphite powder. The prepared catalyst was characterized by transmission electron microscopy, Fourier transform infrared spectroscopy, powder X-ray diffraction, thermogravimetric analysis and ultraviolet–visible spectroscopy. This heterogeneous catalyst was used for the Friedel–Crafts 3-indolylation reaction of isatin with indole derivatives in water as a green media in good to excellent yields. This method has advantages including the use of water as a green solvent, short reaction time, easy workup and excellent yields.









Similar content being viewed by others
References
Cai W, Piner RD, Stadermann FJ, Park S, Shaibat MA, Ishii Y, Yang D, Velamakanni A, An SJ, Stoller M, An J, Chen D, Ruoff RS (2008) Science 321:1815–1817
Xu Y, Bai H, Lu G, Li C, Shi G (2008) J Am Chem Soc 130:5856–5857
Petit C, Bandosz TJ (2009) Adv Funct Mater 21:4753–4757
Karousis N, Economopoulos SP, Sarantopoulou E, Tagmatarchis N (2010) Carbon 48:854–860
Hummers WS, Offeman RE (1958) J Am Chem Soc 80:1339
Szabo T, Tombacz E, Illes E, Dekany I (2006) Carbon 44:537–545
Kim F, Cote LJ, Huang JX (2010) Adv Mater 22:1954–1958
Jia HP, Dreyer DR, Bielawski CW (2011) Tetrahedron 67:4431–4434
Dreyer DR, Jia HP, Bielawski CW (2010) Angew Chem Int Ed 49:6813–6816
Dhakshinamoorthy A, Alvaro M, Concepcio´n P, Forne´s V, Garcia H (2012) Chem Commun 48:5443–5445
Verma S, Mungse HP, Kumar N, Choudhary S, Jain SL, Sain B, Khatri OP (2011) Chem Commun 47:12673–12675
Kumar V, Rao KR (2011) Tetrahedron Lett 52:5188–5191
Liu F, Sun J, Zhu L, Meng X, Qi C, Xiao FS (2012) J Mater Chem 22:5495–5502
Huang J, Zhang L, Chen B, Ji N, Chen F, Zhang Y, Zhang Z (2010) Nanoscale 2:2733–2738
Jasuja K, Linn J, Melten S, Berry V (2010) J Phys Chem Lett 1:1853–1860
Beerthuis R, Granollers M, Brown DR, Salavagione HJ, Rothenberg G, Shiju NR (2015) RSC Adv 5:4103–4108
Scheuermann GM, Rumi L, Steurer P, Bannwarth W, Mulhaupt R (2009) J Am Chem Soc 131:8262–8270
Zhou M, Zhang A, Dai Z, Zhang C, Feng YP (2010) J Chem Phys 132:194704
Stein M, Wieland J, Steurer P, Tolle F, Mulhaupt R, Breit B (2011) Adv Synth Catal 353:523–527
Donner S, Li HW, Yeung ES, Porter MD (2006) Anal Chem 78:2816–2822
Pei S, Cheng H-M (2012) Carbon 50:3210–3228
Jursic BS, Stevens ED (2002) Tetrahedron Lett 43:5681–5683
Klumpp DA, Yeung KY, Prakash GKS, Olah GA (1998) J Org Chem 63:4481–4484
Sharma I, Saxena A, Ojha CK, Paradasani CKP, Paradasani RT, Mukherjee T (2002) Proc Indian Acad Sci (Chem Sci) 114:523–531
Marsden SP, Watson EL, Raw SA (2008) Org Lett 10:2905–2908
Felpin FX, Ibarguren O, Nassar-Hardy L, Fouquet E (2009) J Org Chem 74:1349–1352
Popp FD (1984) J Heterocyclic Chem 21:1367–1368
Garrido F, Ibanez J, Gonalons E, Giraldez A (1975) Eur J Med Chem 10:143–146
Nasseri MA, Allahresani A, Esmaeili AA (2014) Lett Org Chem 11:91–96
Nasseri MA, Allahresani A, Raissi H (2014) Iran J Catal 4:33–40
Nasseri MA, Allahresani A, Raissi H (2014) RSC Adv 4:26087–26093
Allahresani A, Nasseri MA (2014) RSC Adv 4:60702–60710
Kamano Y, Zhang HP, Ichihara Y, Kizu H, Komiyama K, Itokawa H, Pettit GR (1995) Tetrahedron Lett 36:2783–2784
Alinezhad H, Haghighi AH, Salehian F (2010) Chinese Chem Lett 21:183–186
Patel GM, Deota PT (2013) Heterocycl Commun 19:421–424
Karimi AR, Dalirnasab Z, Yousefi GH, Akbarizadeh A (2015) Res Chem Intermed. doi:10.1007/s11164-015-2007-4
Subba Reddy BV, Rajeswari N, Sarangapani M, Prashanthi Y, Ganji RJ, Addlagatta A (2012) Bioorg & Med Chem Lett 22:2460–2463
Rad-Moghadam K, Gholizadeh S (2014) Iran J Catal 4:41–47
Nasseri MA, Ahrari F, Zakerinasab B (2015) RSC Adv 5:26517–26520
Azizian J, Mohammadi AA, Karimi N, Mohammadizadeh MR, Karimi AR (2006) Catal Commun 7:752–755
Acknowledgments
The authors are grateful to the University of Birjand for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allahresani, A., Nasseri, M.A., Akbari, A. et al. Graphene oxide based solid acid as an efficient and reusable nano-catalyst for the green synthesis of diindolyl-oxindole derivatives in aqueous media. Reac Kinet Mech Cat 116, 249–259 (2015). https://doi.org/10.1007/s11144-015-0883-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-015-0883-7