Abstract
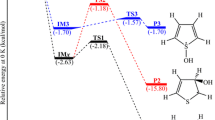
The hydrogenolysis of N,N-dimethyl(3,5-di-tert-butyl-4-hydroxybenzyl)amine, which leads to butylated hydroxytoluene (BHT) as the main product, has been investigated by the density functional theory (DFT) method with the BPE functional and the SVP basis set. A set of valid reaction paths was determined from both thermodynamic and kinetic points of view. Possible products of the reaction were identified, it was shown that the most probable by-product is 4,4′-ethylenebis(2,6-di-tert-butylphenol). The standard gas-phase enthalpies of formation of some possible products of the reaction have been predicted by using the BPE/SVP approach, coupled with isodesmic reactions.




Similar content being viewed by others
References
Biglova RZ, Syrlubaeva RR, Talipov RF (2010) Investigation of antioxidazing activity of oligodiens and oligoolefins functionalized by sulfur and phenols. Bashkir J Chem 17:96–99
Biglova RZ, Surlubaeva RR, Safiullina AA, Talipov RF (2011) Influence of phenols and sulfur functionilised olefins and dienes on solubilising activity of bis oligobutenilcuccinimide. Bashkir J of Chem 18:124–127
Malshe VC, Elango S, Rane S (2006) Alkylated phenolic resins as antioxidants for rubber. J Appl Polym Sci 100:2649–2651
Goda K, Tanaka M, Murata N (1971) Inhibitory action of phenols and amines in the photo-oxidation of tetralin. J Appl Polym Sci 15:403–410
Zhang X, Yang H, Song Y, Zheng Q (2012) Influence of binary combined systems of antioxidants on the stabilization of peroxide-cured low-density polyethylene. J Appl Polym Sci 126:1885–1894
Fujisawa S, Kadoma Y, Yokoe I (2004) Radical-scavenging activity of butylated hydroxytoluene (BHT) and its metabolites. Chem Phys Lipids 130:189–195
Klein E, Lukeš V, Cibulková Z (2005) On the energetics of phenol antioxidants activity. Pet Coal 47:33–39
Villar LD, Silva RF, Diniz MF, Takahashi MFK, Rezende LC (2010) The role of antioxidant on propellant binder reactivity during thermal aging. J Aerosp Technol Manag 2:163–168
Agrawal M, Kim YT, Tonelli A, Whang HS (2010) Cyclodextrin inclusion complex formation with butylated hydroxytoluene and its application in polyethylene film. J Appl Polym Sci 18:1184–1190
Kent B, Abbås Porterv RS (1976) Polystyrene degradation in model extrusion experiments. J Appl Polym Sci 20:1301–1312
Aardt M, Duncan SE, Long TE, O’Keefe SF, Marcy JE, Sims SR (2004) Effect of antioxidants on oxidative stability of edible fats and oils: thermogravimetric analysis. J Agric Food Chem 52:587–591
Gehlawat JK, Sharma MM (1970) Alkylation of phenols with isobutylene. J Appl Chem 20:93–98
Mcconnell WV, Davis HE (1963) Alkylation of phenols. US Patent 3082258 A, 19 May 1963
Yadav GD, Thorat TS (1996) Kinetics of alkylation of p-cresol with isobutylene catalyzed by sulfated zirconia. Ind Eng Chem Res 35:721–731
Salavatova RM, Nijazov NA, Shurupov OK (2009) Method of producing 2,6-di-tert-butyl-4-methyl-phenol. RU Patent 2404956 C2, 2 Feb 2009
Gorbunov BN, Gurevich YA, Maslov IP (1981) Chemistry and technology of stabilizers of polymeric materials. Khimiya, Moscow
Šmejkal F, Popl M (1978) The analysis of condensation products of 2,6-xylenol and formaldehyde in the presence of cresols. Appl Macromol Chem Phys Angew Makromol Chem 67:175–192
Park D, Kobayashi D, Suh H, Cho Y, Lee A (2012) Synthesis of (3-tert-butyl-4-hydroxy-5-methylphenyl) propionate derivatives and their thermal antioxidation behavior for POM. J Appl Polym Sci 124:1731–1736
Hideo A, Kazuyuki I, Eisuke K, Toshiyuki Y (2001) Use of benzotriazolyl-alkylene bisphenol compounds as antioxidants for organic materials. EU Patent 1077246 A3, 14 Mar 2001
Head-Gordon M, Pople JA, Frisch MJ (1988) MP2 energy evaluation by direct methods. Chem Phys Lett 153:503–506
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Perdew JP, Burke K, Enzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3886
Rassolov V, Pople JA, Ratner M, Redfern PC, Curtiss LA (2001) 6-31G* Basis set for third-row atoms. J Comp Chem 22:976–984
Dunning TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023
Schäfer A, Horn H, Ahlrichs R (1992) Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J Chem Phys 97:2571–2577
Afeefy HY, Liebman JF, Stein SE (2001) NIST Chemistry WebBook, NIST standard reference database number 69. Linstrom PJ, Mallard WG. National Institute of Standards and Technology: Gaithersburg, MD. http://webbook.nist.gov
Neese F, Becker U, Ganyushin D, Hansen A, Liakos DG, Kollmar C, Kossmann S, Petrenko T, Reimann C, Riplinger C, Sivalingam K, Valeev E, Wezisla B, Wennmohs F (2009) ORCA. University of Bonn, Bonn
Redfern PC, Zapol P, Curtiss LA (2000) Assessment of gaussian-3 and density functional theories for enthalpies of formation of C1-C16 Alkanes. J Phys Chem A 104:5850–5854
Wong HW, Nieto JCA, Swihart MT, Broadbelt LJ (2004) Thermochemistry of silicon-hydrogen compounds generalized from quantum chemical calculations. J Phys Chem A 108:874–897
Wheeler SE, Houk KH, Schleyer PR, Allen WD (2009) A hierarchy of homodesmotic reactions for thermochemistry. J Am Chem Soc 131:2547–2560
Shaikhlislamov DS, Talipov MR, Khursan SL (2007) Quantum-chemical and group-additive calculations of the enthalpies of formation of hydroxylamines and oximes. Russ J Phys Chem A 81:235–240
Klein E, Rimarčík J, Lukeš V (2009) DFT/B3LYP study of the o–h bond dissociation enthalpies and proton affinities of para- and meta-substituted phenols in water and benzene. Acta Chimica Slovaca 2:37–51
Dean JA (1998) Lange’s handbook of chemistry. McGraw-Hill Professional, New York
Tramontini M, Angiolini L (1994) Mannich bases-chemistry and uses (new directions in organic & biological chemistry). CRC Press, Boca Raton
Bartok M, Molnar A (1997) Heterogeneous catalytic hydrogenation. In: Patai S (ed) Supplement A3: The chemistry of double-bonded functional groups. Wiley, New York
Kwiatek J, Seyler JK (1974) Catalytic hydrogenation and hydrogenolysis by pentacyanocobaltate (II). In: Luberoff BJ (ed) Homogeneous catalysis. Academic Press, London, pp 207–232 Vol 70
Arends IWCE, Ophorst WR, Louw R, Mulder P (1996) Gas phase hydrogenolysis mediated by activated carbon: monosubstituted benzenes. Carbon 34:581–588
Zhong G, Chan B, Radom L (2009) Low barrier hydrogenolysis of the carbon-heteroatom bond as catalyzed by HAlF(4). Org Lett 11:749–751
Meitzner G, Mykytka WJ, Sinfelt JH (1995) Kinetics of hydrogenolysis of methylamine on a rhodium catalyst. Catal Lett 32:335–344
Denekamp C, Tenetov E, Horev Y (2003) Homolytic cleavages in pyridinium ions, an excited state process. J Am Soc Mass Spectrom 14:790–801
Manaa MR, Fried LE (1998) DFT and ab initio study of the unimolecular decomposition of the lowest singlet and triplet states of nitromethane. J Phys Chem A 102:9884–9889
Chen X, Syrstad EA, Nguyen MT, Gerbaux P, Turecˇek F (2004) Distonic isomers and tautomers of the adenine cation radical in the gas phase and aqueous solution. J Phys Chem A 108:9283–9293
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Syrlybaeva, R.R., Belyaeva, A.S. & Khabibullina, G.A. A theoretical determination of the composition of by-products obtained in the production of butylated hydroxytoluene from 2,6-di-tert-butylphenol. Reac Kinet Mech Cat 114, 41–53 (2015). https://doi.org/10.1007/s11144-014-0775-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-014-0775-2