Abstract
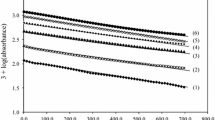
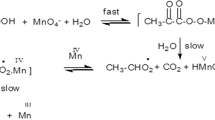
Kinetic investigations on the oxidation of l-asparagine (Asn) by alkaline permanganate have been carried out spectrophotometrically at a constant ionic strength and temperature. The reaction is first order with respect to [MnO4 −] and less than unit order with respect to both [Asn] and [alkali]. The influence of pH indicated that the oxidation is base catalyzed. The reaction rate was found to increase with increasing ionic strength and temperature. The addition of alkali metal ion catalysts accelerates the oxidation rate. The proposed reaction mechanism involves the formation of a 1:1 intermediate complex between l-asparagine and an alkali-permanganate species in a pre-equilibrium step, which was confirmed by both spectral and kinetic evidence. The complex decomposes slowly in a rate determining step, resulting in the formation of a free radical. The latter reacts again with another alkali-permanganate species in a subsequent fast step to yield the final reaction products which were identified as aldehyde (α-formyl acetamide), ammonia, manganate(VI) and carbon dioxide. The appropriate rate laws are deduced. The reaction constants involved in the mechanism were evaluated. The activation and thermodynamic parameters were determined and discussed.












Similar content being viewed by others
References
Sharanabasamma K, Angadi MA, Tuwar SM (2011) Kinetics and mechanism of Ruthenium(III) catalyzed oxidation of l-proline by hexacyanoferrate(III) in aqueous alkali. Open Catal J 4:1–8
Naik KM, Nandibewoor ST (2012) Kinetics and mechanism of oxidation of l-leucine by alkaline diperiodatocuprate(III)-A free radical intervention, deamination and decarboxylation. J Chem Sci 124:809–819
Senagar SKS, Yadav BS (1988) Kinetics and mechanism of copper(II)–catalysed oxidation of asparagine by sodium N-chloro-p-toluene sulphonamide in alkaline media. J Indian Chem Soc 65:88–90
Sanjeevagowda TP, Mahantesh AA, Abdulazizkhan LH (2008) Oxidative deamination and decarboxylation of l-asparagine by the aqueous alkaline diperiodatonickelate(IV) complex. J Solut Chem 37:1795–1808
Cotton FA, Wilkinson G (1980) Advanced inorganic chemistry. Wiley, New York 747
Kembhavi MD, Saleem R, Harihar AL, Nandibewoor ST (2001) Kinetics of oxidative deamination and decarboxylation of l-asparagine by alkaline permanganate: a mechanistic approach. Inorg React Mech 3:39–49
Mahesh RT, Bellakki MB, Nandibewoor ST (2005) Kinetics and mechanism of oxidation of l-proline by heptavalent manganese: a free radical intervention and decarboxylation. J Chem Res 1:13–17
Jose TP, Nandibewoor ST, Tuwar SM (2005) Mechanism of oxidation of l-histidine by heptavalent manganese in alkaline medium. E J Chem 2:75–85
Kini AK, Farokhi SA, Nandibewoor ST (2002) A comparative study of ruthenium(III) catalysed oxidation of l-leucine and l-isoleucine by alkaline permanganate. A kinetic and mechanistic approach. Trans Met Chem 27:532–540
Halligudi LL, Desai SM, Mavalangi AK, Nandibewoor ST (2000) Kinetics of the oxidative degradation of rac-serine by aqueous alkaline permanganate. Mon Fum Chem 131:321–332
Halligudi LL, Desai SM, Mavalangi AK, Nandibewoor ST (2000) Free radical intervention, deamination and decarboxylation in the ruthenium(III)-catalysed oxidation of l-arginine by alkaline permanganate—a kinetic study. Trans Met Chem 26:28–35
Mohanty B, Behera J, Acharya S, Mohanty P, Pantaik AK (2013) Metal ion catalyzed oxidation of l-lysine by alkaline permanganate Ion-A kinetic and mechanistic approach. Chem Sci Trans 2:51–60
Vogel IA (1978) A Text book of quantitative inorganic analysis, 4th edn. ELBS and Longman, New York, p 352
Feigl F (1975) Spot tests in organic analysis. Elsevier, New York 195
Vogel AI (1973) A Text book of practical organic chemistry, 3rd edn. ELBS Longman, London, pp 27–332
Wiberg KB, Deutsch CJ, Rocek J (1973) Permanganate oxidation of crotonic acid. Spectrometric detection of an intermediate. J Am Chem Soc 95:3034–3035
Laider KJ (1965) Chemical kinetics. McGraw-Hill, New York, p 51
Entelis SG, Tiger RP (1976) Reaction kinetics in the liquid phase. Wiley, New York
Ahmed GA, Fawzy A, Hassan RM (2007) Spectrophotometric evidence for the formation of short-lived hypomanganate(V) and manganate(VI) transient species during the oxidation of K-carrageenan by alkaline permanganate. Carbohydr Res 342:1382–1386
Zimmerman CL (1949) Ph D Thesis University of Chicago
Verma RS, Reddy JM, Shastry VR (1974) Kinetic study of homogeneous acid-catalyzed oxidation of certain amino-acids by potassium permanganate in moderately concentrated acidic media. J Chem Soc Perkin Trans 124:469–473
Panari RG, Chougale RB, Nandibewoor ST (1998) Oxidation of mandelic acid by alkaline potassium permanganate. A kinetic study. J Phys Org Chem 11:448–454
De oliveira LA, Toma HE, Giesbrecht E (1976) Kinetics of oxidation of free and coordinated dimethylsulfoxide with permanganate in aqueous solution. Inorg Nucl Chem Lett 2:195–203
Chang R (1981) Physical chemistry with applications to biological systems. MacMillan, New York
Michaelis L, Menten ML (1913) The kinetics of invertase action. Biochem Z 49:333–369
Stewart R (1965) Oxidation in organic chemistry.In: Wiberg KB (ed) Part A, Academic press, New York p 48
Stewart R, Moden RV (1960) The mechanism of the permanganate oxidation of fluoro alcohols in aqueous solution. Disc Faraday Soc 29:211–218
Sathyanarayana DN (2001) Electronic absorption spectroscopy and related techniques. Universities Press, Andhra Pradesh
Hosahalli RV, Savanur AP, Nandibewoor ST, Chimatadar SA (2010) Kinetics and mechanism of uncatalysed and ruthenium(III)-catalysed oxidation of d-panthenol by alkaline permanganate. Trans Met Chem 35:237–246
Weissberger A (1974) Investigation of rates and mechanism of reactions. In: Lewis ES (ed) Techniques of chemistry. Interscience publication, Wiley, p 421
Leffler L, Grunwold E (1963) Rates and equilibria of organic reactions. J Chem Edu 41:407–416
Lewis ES (1974) Investigation of rates and mechanism of reactions, 3rd edn. Wiley, New York, p 415
Exner O (1964) On the enthalpy-entropy relationship. Coll Czech Chem Commun 26:1094–1113
Leffler JE (1955) The enthalpy-entropy relationship for organic chemistry. J Org Chem 20:1202–1231
Lente G, Fabian I, Poe A (2005) A common misconception about the Eyring equation. New J Chem 29:759–760
Duke FR, Parchen RF (1956) The kinetics of the Ce(IV)-Ce(III) exchange reaction in perchloric acid. J Am Chem Soc 78:1540–1543
Taube H, Myers H (1954) Evidence for a bridged activated complex for electron transfer reactions. J Am Chem Soc 76:2103–2111
Wahl AC (1960) Rapid electron-transfer isotopic-exchange reactions. Z Electrochem 64:90–93
Sheppard JC, Wahl AC (1957) Kinetics of the manganate-permanganate exchange reaction. J Am Chem Soc 79:1020–1024
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fawzy, A., Ashour, S.S. & Musleh, M.A. Base-catalyzed oxidation of l-asparagine by alkaline permanganate and the effect of alkali metal ion catalysts: a kinetic and mechanistic approach. Reac Kinet Mech Cat 111, 443–460 (2014). https://doi.org/10.1007/s11144-014-0679-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-014-0679-1