Abstract
Purpose
This study attempted to compare changes in the Quality-of-Life (QoL) scores after three different first-line anti-cancer treatments for advanced non-small cell lung cancer (NSCLC) in a real-world clinical setting.
Patients and methods
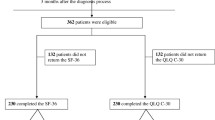
From May 2011 to December 2013, we prospectively measured the QoL scores of patients with locally advanced or metastatic NSCLC using the World Health Organization Quality-of-Life—Brief (WHOQOL-BREF) questionnaire. Each QoL measurement was matched by age and sex with one healthy referent from the National Health Interview Survey. Dynamic changes in patients’ QoL scores and major determinants were repeatedly assessed by construction of a mixed-effects model to adjust for possible confounders.
Results
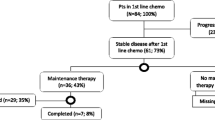
A total of 336 patients with 577 QoL measurements related to first-line anti-cancer treatments were enrolled. Performance status was the most important predictor of QoL scores in all domains after controlling for potential confounders. With age- and sex-matched healthy subjects as the reference, patients treated with gemcitabine + platinum showed significantly lower scores in multiple physical and psychological domain items in the WHOQOL-BREF. However, pemetrexed + platinum and gefitinib/erlotinib affected patients’ QoL scores in ‘energy/fatigue’ and ‘daily activities’ with smaller magnitudes, and the scores appeared to improve after 3–4 months of treatment.
Conclusions
Patients receiving gemcitabine + platinum as first-line anti-cancer treatment for advanced NSCLC experienced relatively poor QoL scores throughout treatment course. Studies to develop a real-time computerized system automatically updating the mixed-effects model for QoL to facilitate participatory clinical decision making by physicians, patients, and their families merit further research.

Similar content being viewed by others
References
Ferlay, J., Shin, H. R., Bray, F., Forman, D., Mathers, C., & Parkin, D. M. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer, 127, 2893–2917.
American Cancer Society. (2010). Cancer Facts and Figures 2010. Atlanta: American Cancer Society.
Claassens, L., van Meerbeeck, J., Coens, C., Quinten, C., Ghislain, I., Sloan, E. K., et al. (2011). Health-related quality of life in non-small-cell lung cancer: An update of a systemic review on methodological issues in randomized controlled trials. Journal of Clinical Oncology, 29, 2104–2120.
Lee, L. J., Chung, C. W., Chang, Y. Y., Lee, Y. C., Yang, C. H., Liou, S. H., et al. (2011). Comparison of the quality of life between patients with non-small-cell lung cancer and healthy controls. Quality of Life Research, 20, 415–423.
Lin, C. Y., Yang, S. C., Lai, W. W., Su, W. C., & Wang, J. D. (2015). Rasch models suggested the satisfactory psychometric properties of the World Health Organization Quality of Life—Brief among lung cancer patients. Journal of Health Psychology,. doi:10.1177/1359105315603474.
Chen, G., Feng, J., Zhou, C., Wu, Y. L., Liu, X. Q., Wang, C., et al. (2013). Quality of life (QoL) analyses from OPTIMAL (CTONG-0802), a phase III, randomized, open-label study of first-line erlotinib versus chemotherapy in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC). Annals of Oncology, 24, 1615–1622.
Grønberg, B. H., Bremnes, R. M., Fløtten, Ø., Amundsen, T., Brunsvig, P. F., Hjelde, H. H., et al. (2009). Phase III study by the Norwegian lung cancer study group: Pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. Journal of Clinical Oncology, 27, 3217–3224.
Tazaki, M., Nakane, Y., Endo, T., Kakikawa, F., Kano, K., Kawano, H., et al. (1998). Results of a qualitative and field study using the WHOQOL instrument for cancer patients. Japanese Journal of Clinical Oncology, 28, 134–141.
Mohan, A., Mohan, C., Pathak, A. K., Pandey, R. M., & Guleria, R. (2007). Impact of chronic obstructive pulmonary disease on respiratory status and quality of life in newly diagnosed patients with lung cancer. Respirology, 12, 240–247.
Yao, G., Chung, C. W., Yu, C. F., & Wang, J. D. (2002). Development and verification of validity and reliability of the WHOQOL-BREF Taiwan version. Journal of the Formosan Medical Association, 101, 342–351.
The WHOQOL Group, Programme on Mental Health, WHO. (1996). WHOQOL-BREF introduction, administration, scoring and generic version of the assessment. Geneva, Switzerland: World Health Organization.
Rosell, R., Carcereny, E., Gervais, R., Vergnenegre, A., Massuti, B., Felip, E., et al. (2012). Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncology, 13, 239–246.
Leighl, N. B. (2012). Treatment paradigms for patients with metastatic non-small-cell lung cancer: First-, second-, and third- line. Current Oncology, 19, S52–S58.
Hwang, J. S., & Wang, J. D. (2004). Integrating health profile with survival for quality of life assessment. Quality of Life Research, 13, 1–10.
Phungrassami, T., Katikarn, R., Watanaarepornchai, S., & Sangtawan, D. (2004). Quality of life assessment in radiotherapy patients by WHOQOL-BREF-THAI: A feasibility study. Journal of the Medical Association of Thailand, 87, 1459–1465.
Zimmermann, C., Burman, D., Swami, N., Krzyzanowska, M. K., Leighl, N., Moore, M., et al. (2011). Determinants of quality of life in patients with advanced cancer. Supportive Care in Cancer, 19, 621–629.
Lam, K., Chow, E., Zhang, L., Wong, E., Bedard, G., Fairchild, A., et al. (2013). Determinants of quality of life in advanced cancer patients with bone metastases undergoing palliative radiation treatment. Supportive Care in Cancer, 21, 3021–3030.
Jatoi, A., Novotny, P., Cassivi, S., Clark, M. M., Midthun, D., Patten, C. A., et al. (2007). Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the Mayo Clinic lung cancer cohort. Oncologist, 12, 1456–1463.
Scagliotti, G. V., Parikh, P., von Pawel, J., Biesma, B., Vansteenkiste, J., Manegold, C., et al. (2008). Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. Journal of Clinical Oncology, 26, 3543–3551.
Scagliotti, G. V., Park, K., Patil, S., Rolski, J., Goksel, T., Martins, R., et al. (2009). Survival without toxicity for cisplatin plus pemetrexed versus cisplatin plus gemcitabine in chemonaive patients with advanced non-small cell lung cancer: a risk-benefit analysis of a large phase III study. European Journal of Cancer, 45, 2298–2303.
Han, J. Y., Park, K., Kim, S. W., Lee, D. H., Kim, H. Y., Kim, H. T., et al. (2012). First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. Journal of Clinical Oncology, 30, 1122–1128.
Zhou, C., Wu, Y. L., Chen, G., Feng, J., Liu, X. Q., Wang, C., et al. (2011). Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicenter, open-label, randomized, phase 3 study. Lancet Oncology, 12, 735–742.
Wang, B. Y., Huang, J. Y., Cheng, C. Y., Lin, C. H., Ko, J., & Liaw, Y. P. (2013). Lung cancer and prognosis in Taiwan: A population-based cancer registry. Journal of Thoracic Oncology, 8, 1128–1135.
Sloan, J. A., Zhao, X., Novotny, P. J., Wampfler, J., Garces, Y., Clark, M. M., et al. (2012). Relationship between deficits in overall quality of life and non-small-cell lung cancer survival. Journal of Clinical Oncology, 30, 1498–1504.
Chen, J., Qi, Y., Wampfler, J. A., Jatoi, A., Garces, Y. I., Busta, A. J., et al. (2012). Effect of cigarette smoking on quality of life in small cell lung cancer patients. European Journal of Cancer, 48, 1593–1601.
Acknowledgments
This research was supported, in part, by the Ministry of Science and Technology [NSC102-2314-B-006-029-MY2]; the Ministry of Health and Welfare [DOH100-TD-C-111-003 to W.W. Lai, MOHW103-TD-B-111-06 and MOHW103-TDU-B-211-113002 to W.C. Su]; and the Ministry of Education, Taiwan, R.O.C., the Aim for the Top University Project to National Cheng Kung University. We thank the help from Cancer Data Bank of National Cheng Kung University Hospital for retrieving the cancer case database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The original study was approved by the Institutional Review Board at National Cheng Kung University Hospital. All participants signed informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, SC., Lai, WW., Hsiue, TR. et al. Health-related quality of life after first-line anti-cancer treatments for advanced non-small cell lung cancer in clinical practice. Qual Life Res 25, 1441–1449 (2016). https://doi.org/10.1007/s11136-015-1174-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-015-1174-5