Abstract
Purpose
In advanced non-small cell lung cancer (NSCLC), progressive disease burdens patients considerably. Second-line (2L) chemotherapy improves survival marginally but humanistic outcomes (i.e., quality of life, QOL) are underreported. The impact of 2L therapy remains an important consideration for patients and caregivers, and there have been QOL reviews for 1L, but not 2L, therapies. This review assessed QOL outcomes of approved, guideline-supported 2L chemotherapy with docetaxel, erlotinib, gefitinib, and pemetrexed in advanced NSCLC.
Methods
Clinical trial reports of approved, guideline-supported 2L or maintenance therapy for NSCLC published from 2000 to 2010 were identified from PubMed/Medline and clinical meetings. Outcomes were stratified by overall QOL impact, domain/symptom-specific effects, effect over time, and subgroup effects.
Results
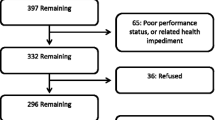
Of 145 studies identified, 24 full-text articles were retained. Studies with docetaxel versus best supportive care (n = 1) and active comparators (n = 4) reported non-significant overall QOL improvements, as did studies of gefitinib versus placebo and active comparator (n = 7). Overall QOL improvements were seen for gefitinib versus docetaxel (n = 2) and gefitinib in a single-arm study (n = 1). At the symptom level, studies of docetaxel (n = 4/7), gefitinib (n = 7/9), and pemetrexed (n = 1) reported non-significant results. Subgroup analyses indicated improved QOL outcomes for gefitinib-treated responders versus non-responders, worse QOL for gefitinib-treated smokers versus placebo, worse QOL for gefitinib-treated Asian patients versus placebo, and longer time to symptom deterioration in erlotinib versus placebo-treated elderly patients.
Conclusions
Significant improvements in overall QOL with 2L chemotherapy for advanced NSCLC were infrequent. Single-arm studies and those with less toxic regimens more commonly provided statistically significant improvements in QOL outcomes. Methodological heterogeneity impedes cross-study QOL comparisons.

Similar content being viewed by others
References
Wisnivesky, J. P., Yankelevitz, D., & Henschke, C. I. (2005). Stage of lung cancer in relation to its size: Part 2. Evidence. Chest, 127(4), 1136–1139.
Azzoli, C. G., Temin, S., Aliff, T., Baker, S., Jr, Brahmer, J., Johnson, D. H., et al. (2011). 2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. Journal of Clinical Oncology, 29(28), 3825–3831.
NCCN Guidelines Panel. (2012). NCCN Clinical practice guidelines in oncology: Non small cell lung cancer: Version 3.2012.
Goffin, J., Lacchetti, C., Ellis, P. M., Ung, Y. C., & Evans, W. K. (2010). First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: A systematic review. Journal of Thoracic Oncology, 5(2), 260–274.
Stinchcombe, T. E., & Socinski, M. A. (2008). Considerations for second-line therapy of non-small cell lung cancer. Oncologist, 13(Suppl), 128–136.
Schiller, J. H., Harrington, D., Belani, C. P., Langer, C., Sandler, A., Krook, J., et al. (2002). Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. New England Journal of Medicine, 346(2), 92–98.
Sandler, A., Gray, R., Perry, M. C., Brahmer, J., Schiller, J. H., Dowlati, A., et al. (2006). Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. New England Journal of Medicine, 355(24), 2542–2550.
Socinski, M. A., Schell, M. J., Peterman, A., Bakri, K., Yates, S., Gitten, R., et al. (2002). Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. Journal of Clinical Oncology, 20(5), 1335–1343.
Passaro, A., Cortesi, E., & De, M. F. (2011). Second-line treatment of non-small-cell lung cancer: Chemotherapy or tyrosine kinase inhibitors? Expert Review of Anticancer Therapy, 11(10), 1587–1597.
Maione, P., Rossi, A., Bareschino, M. A., Sacco, P. C., Schettino, C., Falanga, M., et al. (2011). Factors driving the choice of the best second-line treatment of advanced NSCLC. Reviews on Recent Clinical Trials, 6(1), 44–51.
Herbst, R. S., Prager, D., Hermann, R., Fehrenbacher, L., Johnson, B. E., Sandler, A., et al. (2005). TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. Journal of Clinical Oncology, 23(25), 5892–5899.
Hanna, N., Shepherd, F. A., Fossella, F. V., Pereira, J. R., De, M. F., Von Pawel, J., et al. (2004). Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. Journal of Clinical Oncology, 22(9), 1589–1597.
Fossella, F., Pereira, J. R., Pawel von, P. J., Pluzanska, A., Gorbounova, V., Kaukel, E., et al. (2003). Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: The TAX 326 study group. Journal of Clinical Oncology, 21(16), 3016–3024.
Moro-Sibilot, D., Vergnenegre, A., Smit, E. F., Toy, E., Parente, B., Schmitz, S., et al. (2010). Second-line therapy for NSCLC in clinical practice: Baseline results of the European SELECTION observational study. Current Medical Research and Opinion, 26(11), 2661–2672.
Silvestri, G., Pritchard, R., & Welch, H. G. (1998). Preferences for chemotherapy in patients with advanced non-small cell lung cancer: Descriptive study based on scripted interviews. BMJ, 317(7161), 771–775.
Dancey, J., Shepherd, F. A., Gralla, R. J., & Kim, Y. S. (2004). Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: Results of a prospective, randomized phase III trial. Lung Cancer, 43(2), 183–194.
Gebbia, V., Gridelli, C., Verusio, C., Frontini, L., Aitini, E., Daniele, B., et al. (2009). Weekly docetaxel vs. docetaxel-based combination chemotherapy as second-line treatment of advanced non-small-cell lung cancer patients. The DISTAL-2 randomized trial. Lung Cancer, 63(2), 251–258.
Bezjak, A., Tu, D., Seymour, L., Clark, G., Trajkovic, A., Zukin, M., et al. (2006). Symptom improvement in lung cancer patients treated with erlotinib: Quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. Journal of Clinical Oncology, 24(24), 3831–3837.
Kim, E. S., Hirsh, V., Mok, T., Socinski, M. A., Gervais, R., Wu, Y. L., et al. (2008). Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): A randomised phase III trial. Lancet, 372(9652), 1809–1818.
Tanvetyanon, T., Soares, H. P., Djulbegovic, B., Jacobsen, P. B., & Bepler, G. (2007). A systematic review of quality of life associated with standard chemotherapy regimens for advanced non-small cell lung cancer. Journal of Thoracic Oncology, 2(12), 1091–1097.
D’Addario, G., Fruh, M., Reck, M., Baumann, P., Klepetko, W., & Felip, E. (2010). Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 21(Suppl 5), v116–v119.
Gridelli, C., Maione, P., Rossi, A., Ferrara, M. L., Bareschino, M. A., Schettino, C., et al. (2009). Potential treatment options after first-line chemotherapy for advanced NSCLC: Maintenance treatment or early second-line? Oncologist, 14(2), 137–147.
Committee For Medicinal Products For Human Use. (2005). Reflection Paper On The Regulatory Guidance For The Use Of Healthrelated Quality Of Life (Hrql) Measures In The Evaluation Of Medicinal Products.25.
US Department of Health and Human Services. (2009). Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims.43.
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2010). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International Journal of Surgery, 8(5), 336–341.
National Institutes of Health (2012). ClinicalTrials.gov.
Efficace, F., Bottomley, A., Osoba, D., Gotay, C., Flechtner, H., D’haese, S., et al. (2003). Beyond the development of health-related quality-of-life (HRQOL) measures: A checklist for evaluating HRQOL outcomes in cancer clinical trials–does HRQOL evaluation in prostate cancer research inform clinical decision making? Journal of Clinical Oncology, 21(18), 3502–3511.
US Food an Drug Administration (2003). Workshop summary on endpoints for approval of cancer drugs for lung cancer.
Fidias, P. M., Dakhil, S. R., Lyss, A. P., Loesch, D. M., Waterhouse, D. M., Bromund, J. L., et al. (2009). Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. Journal of Clinical Oncology, 27(4), 591–598.
Gridelli, C., Gallo, C., Di, M. M., Barletta, E., Illiano, A., Maione, P., et al. (2004). A randomised clinical trial of two docetaxel regimens (weekly vs. 3 week) in the second-line treatment of non-small-cell lung cancer. The DISTAL 01 study. British Journal of Cancer, 91(12), 1996–2004.
Krzakowski, M., Ramlau, R., Jassem, J., Szczesna, A., Zatloukal, P., Von Pawel, J., et al. (2010). Phase III trial comparing vinflunine with docetaxel in second-line advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy. Journal of Clinical Oncology, 28(13), 2167–2173.
Lai, C. L., Tsai, C. M., Chiu, C. H., Wang, G. S., Su, W. J., Chen, Y. M., et al. (2005). Phase II randomized trial of tri-weekly versus days 1 and 8 weekly docetaxel as a second-line treatment of advanced non-small cell lung cancer. Japanese Journal of Clinical Oncology, 35(12), 700–706.
Park, J. O., Kim, S. W., Ahn, J. S., Suh, C., Lee, J. S., Jang, J. S., et al. (2007). Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non small-cell lung cancer. Journal of Clinical Oncology, 25(33), 5233–5239.
Paz-Ares, L., Ross, H., O’Brien, M., Riviere, A., Gatzemeier, U., von Pawel, J., et al. (2008). Phase III trial comparing paclitaxel poliglumex vs docetaxel in the second-line treatment of non-small-cell lung cancer. British Journal of Cancer, 98(10), 1608–1613.
Wheatley-Price, P., Ding, K., Seymour, L., Clark, G. M., & Shepherd, F. A. (2008). Erlotinib for advanced non-small-cell lung cancer in the elderly: An analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. Journal of Clinical Oncology, 26(14), 2350–2357.
Cappuzzo, F., Ciuleanu, T., Stelmakh, L., Cicenas, S., Szczesna, A., Juhasz, E., et al. (2010). Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncology, 11(6), 521–529.
Perez-Soler, R., Chachoua, A., Hammond, L. A., Rowinsky, E. K., Huberman, M., Karp, D., et al. (2004). Determinants of tumor response and survival with erlotinib in patients with non–small-cell lung cancer. Journal of Clinical Oncology, 22(16), 3238–3247.
Cella, D., Herbst, R. S., Lynch, T. J., Prager, D., Belani, C. P., Schiller, J. H., et al. (2005). Clinically meaningful improvement in symptoms and quality of life for patients with non-small-cell lung cancer receiving gefitinib in a randomized controlled trial. Journal of Clinical Oncology, 23(13), 2946–2954.
Fukuoka, M., Yano, S., Giaccone, G., Tamura, T., Nakagawa, K., Douillard, J. Y., et al. (2003). Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. Journal of Clinical Oncology, 21(12), 2237–2246.
Gelibter, A., Ceribelli, A., Pollera, C. F., Milella, M., Moscetti, L., Sperduti, I., et al. (2005). Impact of gefitinib (‘Iressa’) treatment on the quality of life of patients with advanced non-small-cell lung cancer. Journal of Cancer Research Clinical Oncology, 131(12), 783–788.
Kris, M. G., Natale, R. B., Herbst, R. S., Lynch, T. J., Jr., Prager, D., Belani, C. P., et al. (2003). Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA, 290(16), 2149–2158.
Lee, D. H., Park, K., Kim, J. H., Lee, J. S., Shin, S. W., Kang, J. H., et al. (2010). Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clinical Cancer Research, 16(4), 1307–1314.
Mu, X. L., Li, L. Y., Zhang, X. T., Wang, S. L., & Wang, M. Z. (2004). Evaluation of safety and efficacy of gefitinib (‘iressa’, zd1839) as monotherapy in a series of Chinese patients with advanced non-small-cell lung cancer: Experience from a compassionate-use programme. BMC Cancer, 4, 51.
Sekine, I., Ichinose, Y., Nishiwaki, Y., Yamamoto, N., Tsuboi, M., Nakagawa, K., et al. (2009). Quality of life and disease-related symptoms in previously treated Japanese patients with non-small-cell lung cancer: Results of a randomized phase III study (V-15–32) of gefitinib versus docetaxel. Annals of Oncology, 20(9), 1483–1488.
Takeda, K., Hida, T., Sato, T., Ando, M., Seto, T., Satouchi, M., et al. (2010). Randomized phase III trial of platinum-doublet chemotherapy followed by gefitinib compared with continued platinum-doublet chemotherapy in Japanese patients with advanced non-small-cell lung cancer: Results of a west Japan thoracic oncology group trial (WJTOG0203). Journal of Clinical Oncology, 28(5), 753–760.
Thatcher, N., Chang, A., Parikh, P., Rodrigues, P. J., Ciuleanu, T., von Pawel, J., et al. (2005). Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet, 366(9496), 1527–1537.
Cufer, T., Vrdoljak, E., Gaafar, R., Erensoy, I., & Pemberton, K. (2006). Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer. Anti-Cancer Drugs, 17(4), 401–409.
De, M. F., Pereira, J. R., Fossella, F., Perry, M. C., Reck, M., Salzberg, M., et al. (2008). Lung Cancer Symptom Scale outcomes in relation to standard efficacy measures: An analysis of the phase III study of pemetrexed versus docetaxel in advanced non-small cell lung cancer. Journal of Thoracic Oncology, 3(1), 30–36.
Plunkett, T. A., Chrystal, K. F., & Harper, P. G. (2003). Quality of life and the treatment of advanced lung cancer. Clinical Lung Cancer, 5(1), 28–32.
Oort, F. J., Visser, M. R., & Sprangers, M. A. (2009). Formal definitions of measurement bias and explanation bias clarify measurement and conceptual perspectives on response shift. Journal of Clinical Epidemiology, 62(11), 1126–1137.
Acknowledgments
P.W., X.G., J.A.C., and M.F.B. are employees of Pharmerit International, which received funding support related to the development of this manuscript from Abbott Laboratories. A.G. and S.R. are employees of Abbott Laboratories.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ganguli, A., Wiegand, P., Gao, X. et al. The impact of second-line agents on patients’ health-related quality of life in the treatment for non-small cell lung cancer: a systematic review. Qual Life Res 22, 1015–1026 (2013). https://doi.org/10.1007/s11136-012-0229-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-012-0229-0