Abstract
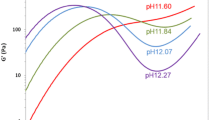
Heat-denaturation of quinoa protein isolate (QPI) at alkali pH and its influence on the physicochemical and cold gelation properties was investigated. Heating QPI at pH 8.5 led to increased surface hydrophobicity and decreases in free and bound sulfhydryl group contents. Heating at pH 10.5 caused a lesser degree of changes in sulfhydryl groups and surface hydrophobicity, and the resulting solutions showed drastically increased solubility. SDS PAGE revealed the presence of large aggregates only in the sample heated at pH 8.5, suggesting that any aggregates present in the sample heated at pH 10.5 were non-covalently bound and disintegrated in the presence of SDS. Reducing conditions partially dissolved the aggregates in the pH 8.5 heated sample indicating the occurrence of disulphide bonding, but caused no major alterations in the separation pattern of the pH 10.5 heated sample. Denaturation pH influenced the cold gelation properties greatly. Solutions heated at pH 8.5 formed a coarse coagulum with maximum G’ of 5 Pa. Heat-denaturation at 10.5 enabled the proteins to form a finer and regularly structured gel with a maximum G’ of 1140 Pa. Particle size analysis showed that the pH 10.5 heated sample contained a higher level of very small particles (0.1–2 μm), and these readily aggregated into large particles (30–200 μm) when pH was lowered to 5.5. Differences in the nature of aggregates formed during heating may explain the large variation in gelation properties.





Similar content being viewed by others
Abbreviations
- ANS:
-
1-anilino-8-naphthalene sulfonate
- GDL:
-
D-Gluconic acid δ-lactone (GDL)
- QPI:
-
Quinoa protein isolate
- SDS PAGE:
-
Sodium dodecyl sulphate polyacrylamide gel electrophoresis
- TNB:
-
5,5′-dithio-bis-(2-nitrobenzoic acid)
References
Day L (2013) Proteins from land plants–potential resources for human nutrition and food security. Trends Food Sci Technol 32:25–42. doi:10.1016/j.tifs.2013.05.005
Ruales J, Nair BM (1992) Nutritional quality of the protein in quinoa (Chenopodium quinoa Willd.) seeds. Plant Foods Hum Nutr 42:1–11. doi:10.1007/BF02196067
Peñas E, Uberti F, di Lorenzo C, Ballabio C, Brandolini A, Restani P (2014) Biochemical and immunochemical evidences supporting the inclusion of quinoa (Chenopodium quinoa Willd.) as a gluten-free ingredient. Plant Foods Hum Nutr 69:297–303. doi:10.1007/s11130-014-0449-2
Brinegar C, Goundan S (1993) Isolation and characterization of chenopodin, the 11S seed storage protein of quinoa (Chenopodium quinoa). J Agric Food Chem 41:182–185. doi:10.1021/jf00026a006
Brinegar C, Sine B, Nwokocha L (1996) High-cysteine 2S seed storage proteins from quinoa (Chenopodium quinoa). J Agric Food Chem 44:1621–1623. doi:10.1021/jf950830+
Prego I, Maldonado S, Otegui M (1998) Seed structure and localization of reserves in Chenopodium quinoa. Ann Bot 82:481–488. doi:10.1006/anbo.1998.0704
Ando H, Chen Y-C, Tang H, Shimizu M, Watanabe K, Mitsunaga T (2002) Food components in fractions of quinoa seed. Food Sci Technol Res 8:80–84. doi:10.3136/fstr.8.80
Foegeding EA, Davis JP (2011) Food protein functionality: a comprehensive approach. Food Hydrocoll 25:1853–1864. doi:10.1016/j.foodhyd.2011.05.008
Hermansson A-M (1979) Aggregation and denaturation involved in gel formation - Chapter 5. In: Pour-El A (ed) Functionality and protein structure. American Chemical Society, Washington DC, pp 281–304. doi:10.1021/bk-1979-0092.ch005
Alting AC, Weijers M, de Hoog EHA, van de Pijpekamp AM, Cohen Stuart MA, Hamer RJ, Visschers RW (2004) Acid-induced cold gelation of globular proteins: effects of protein aggregate characteristics and disulfide bonding on rheological properties. J Agric Food Chem 52:623–631. doi:10.1021/jf034753r
Boye JI, Alli I, Ismail AA, Gibbs BF, Konishi Y (1995) Factors affecting molecular characteristics of whey protein gelation. Int Dairy J 5:337–353. doi:10.1016/0958-6946(94)00012-E
Bryant CM, McClements DJ (1998) Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci Technol 9:143–151. doi:10.1016/S0924-2244(98)00031-4
Abugoch LE, Romero N, Tapia CA, Silva J, Rivera M (2008) Study of some physicochemical and functional properties of quinoa (Chenopodium quinoa Willd.) protein isolates. J Agric Food Chem 56:4745–4750. doi:10.1016/S1043-4526(09)58001-1
EEC (2009) Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off J Eur Union L 054, 26.2.2009, pp 1
Wolf WJ, Tamura T (1969) Heat denaturation of soybean IIS protein. Cereal Chem 46:331–344
Monahan FJ, German JB, Kinsella JE (1995) Effect of pH and temperature on protein unfolding and thiol/disulfide interchange reactions during heat-induced gelation of whey proteins. J Agric Food Chem 43:46–52. doi:10.1021/jf00049a010
Mäkinen OE, Uniacke-Lowe T, O’Mahony JA, Arendt EK (2015) Physicochemical and acid gelation properties of commercial UHT-treated plant-based milk substitutes and lactose free bovine milk. Food Chem 168:630–638. doi:10.1016/j.foodchem.2014.07.036
Westermeier R (2006) Sensitive, quantitative, and fast modifications for Coomassie Blue staining of polyacrylamide gels. Proteomics 6(S2):61–64. doi:10.1002/pmic.200690121
Whitaker JR, Feeney RE (1983) Chemical and physical modification of proteins by the hydroxide ion. Crit Rev Food Sci Nutr 19:173–212. doi:10.1080/10408398309527375
Wang CH, Damodaran S (1990) Thermal destruction of cysteine and cystine residues of soy protein under conditions of gelation. J Food Sci 55:1077–1080. doi:10.1111/j.1365-2621.1990.tb01602.x
Riha WE, Izzo HV, Zhang J, Ho CT (1996) Nonenzymatic deamidation of food proteins. Crit Rev Food Sci Nutr 36:225–255. doi:10.1080/10408399609527724
German B, Damodaran S, Kinsella JE (1982) Thermal dissociation and association behavior of soy proteins. J Agric Food Chem 30:807–811. doi:10.1021/jf00113a002
Marcone MF (1999) Biochemical and biophysical properties of plant storage proteins. Food Res Int 32:79–92. doi:10.1016/S0963-9969(99)00061-7
Hermansson AM (1986) Soy protein gelation. J Am Oil Chem Soc 63:658–666. doi:10.1007/BF02638232
Renkema JM, Lakemond CM, de Jongh HH, Gruppen H, van Vliet T (2000) The effect of pH on heat denaturation and gel forming properties of soy proteins. J Biotechnol 79:223–230. doi:10.1016/S0168-1656(00)00239-X
Doucet D, Gauthier SF, Foegeding EA (2001) Rheological characterization of a gel formed during extensive enzymatic hydrolysis. J Food Sci 66:711–715. doi:10.1111/j.1365-2621.2001.tb04626.x
Kuipers BJH, Gruppen H (2008) Identification of strong aggregating regions in soy glycinin upon enzymatic hydrolysis. J Agric Food Chem 56:3818–3827. doi:10.1021/jf703781j
Acknowledgments
Outi Mäkinen was funded by the Food Institutional Research Measure administered by the Department of Agriculture, Fisheries and Food (Ireland). The authors wish to express their sincere gratitude to Walter von Reding, Uwe Schill and Uncas Roukema from Bühler AG for the fractionation of quinoa.
Conflict of Interest
Outi E. Mäkinen has no conflict of Interest.
Emanuele Zannini has no conflict of interest.
Elke K. Arendt has no conflict of Interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mäkinen, O.E., Zannini, E. & Arendt, E.K. Modifying the Cold Gelation Properties of Quinoa Protein Isolate: Influence of Heat-Denaturation pH in the Alkaline Range. Plant Foods Hum Nutr 70, 250–256 (2015). https://doi.org/10.1007/s11130-015-0487-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-015-0487-4