Abstract
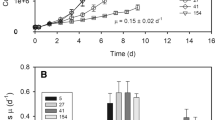
Photoacclimation was studied in Thalassiosira pseudonana to help understand mechanisms underlying the success of diatoms in low-light environments, such as coastal and deep mixing ecosystems. Light harvesting and other cell characteristics were combined with oxygen and carbon production measurements to assess the water-splitting reaction at PSII (\({\text{GPP}}_{{{\text{O}}_{2} }}\)) and intermediate steps leading to net carbon production (NPPC). These measurements revealed that T. pseudonana is remarkably efficient at converting harvested light energy into biomass, with at least 57 % of \({\text{GPP}}_{{{\text{O}}_{2} }}\) retained as NPPC across all light-limited growth rates examined. Evidence for upregulation of ATP generation pathways that circumvent carbon fixation indicated that high growth efficiency at low light levels was at least partly due to increases in the efficiency of ATP production. Growth rate-dependent demands for ATP and NADPH were reflected in carbon composition and in unexpected shifts in the light-limited slope (α) of photosynthesis–irradiance relationships generated from chlorophyll-specific 14C-uptake. Overall, these results suggest that pathway gating of carbon and energy flow depends on light availability and is a key factor promoting the efficiency of diatom growth at low light intensities.



Similar content being viewed by others
References
Allen JF (2003) Cyclic, pseudocyclic and noncyclic photophosphorylation: new links in the chain. Trends Plant Sci 8(1):15–19
Allen AE, La Roche J, Maheswari U, Lommer M, Schauer N, Lopez PJ, Finazzi G, Fernie AR, Bowler C (2008) Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proc Natl Acad Sci 105(30):10438–10443
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Bailleul B, Berne N, Murik O, Petroutsos D, Prihoda J, Tanaka A, Villanova V, Bligny R, Flori S, Falconet D, Drieger-Liszkay A, Santabarbara S, Rappaport F, Joliot P, Tirichine L, Falkowski P, Cardol P, Bowler C, Finazzi G (2015) Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature 524:366–369
Bartual A, Gálvez JA (2002) Growth and biochemical composition of the diatom Phaeodactylum tricornutum at different pH and inorganic carbon levels under saturating and subsaturating light regimes. Bot Mar 45(6):491–501
Bate GC, Sueltemeyer DF, Fock HP (1988) 16O2/18O2 analysis of oxygen exchange in Dunaliella tertiolecta. Evidence for the inhibition of mitochondrial respiration in the light. Photosynth Res 16:219–231
Behrenfeld MJ, Prasil O, Babin M, Bruyant F (2004) In search of a physiological basis for covariations in light-limited and light-saturated photosynthesis. J Phycol 40:4–25
Behrenfeld MJ, Halsey KH, Milligan AJ (2008) Evolved physiological responses of phytoplankton to their integrated growth environment. Philos Trans R Soc Lond B 363(1504):2687–2703
Berges JA, Falkowski PG (1998) Physiological stress and cell death in marine phytoplankton: induction response to nitrogen or light limitation. Limnol Oceanogr 43(1):129–135
Bittar TB, Lin Y, Sassano LR, Wheeler BJ (2013) Carbon allocation under light and nitrogen resource gradients in two model marine phytoplankton. J Phycol 49:523–535
Bowler C, De Martino A, Falciatore A (2010) Diatom cell division in an environmental context. Curr Opin Plant Biol 13:623–0630
Crombet Y, Leblanc K, Quéguiner B, Moutin T, Rimmelin P, Ras J, Claustre H, Leblond N, Orio L, Pujo-Pay M (2011) Deep silicon maxima in the stratified oligotrophic Mediterranean Sea. Biogeosciences 8:459–475
Dubinsky Z, Falkowski PG, Post AF, van Hes UM (1987) A system for measuring phytoplankton photosynthesis in a defined light field with an oxygen electrode. J Plankton Res 9:607–612
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281(5374):237–240
Fietz S, Nicklisch A (2002) Acclimation of the diatom Stephanodiscus neoastraea and the cyanobacterium Planktothrix agardhii to simulated natural light fluctuations. Photosynth Res 72:95–106
Flynn KJ (2001) A mechanistic model for describing dynamic multi-nutrient, light, temperature interactions in phytoplankton. J Plankton Res 23(9):977–997
Furnas MJ (1990) In situ growth rates of marine phytoplankton: approaches to measurement, community and species growth rate. J Plankton Res 12:1117–1151
Geider RJ (1987) Light and temperature dependence of the carbon to chlorphyll ratio in microalgae and cyanobacteria: implications for physiology and growth of phytoplankton. New Phytol 106:1–34
Geider RJ, Osborne BA, Raven JA (1985) Light dependence of growth and photosynthesis in Phaeodactylum tricornutum (Bacillariophyceae). J Phycol 21:609–619
Geider RJ, MacIntyre HL, Kana TM (1997) Dynamic model of phytoplankton growth and acclimation: responses of the balanced growth rate and the chlorphyll a:carbon ratio to light, nutrient-limitation and temperature. Mar Ecol Prog Ser 148(1–3):187–200
Geider RJ, MacIntyre HL, Kana TM (1998) A dynamic regulatory model of phytoplankton acclimation to light, nutrients, and temperature. Limnol Oceanogr 43(4):679–694
Geider RJ, Moore CM, Ross ON (2009) The role of cost-benefit analysis in models of phytoplankton growth and acclimation. Plant Ecol Divers 2(2):165–178
Granum E, Kirkvold S, Myklestad SM (2002) Cellular and extracellular production of carbohydrates and amino acids by the marine diatom Skeletonema costatum: diel variations and effects of N depletion. Mar Ecol Prog Ser 242:83–94
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 26–60
Halsey KH, Jones BM (2015) Phytoplankton strategies for photosynthetic energy allocation. Annu Rev Mar Sci 7:265–297
Halsey KH, Milligan AJ, Behrenfeld MJ (2010) Physiological optimization underlies growth rate-independent chlorophyll-specific gross and net primary production. Photosynth Res 103(2):125–137
Halsey KH, Milligan AJ, Behrenfeld MJ (2011) Linking time-dependent carbon-fixation efficiencies in Dunaliella tertiolecta (Chlorophyceae) to underlying metabolic pathways. J Phycol 47(1):66–76
Halsey KH, O’Malley RT, Graff JR, Milligan AJ, Behrenfeld MJ (2013) A common partitioning strategy for photosynthetic products in evolutionarily distinct phytoplankton species. New Phytol 198(4):1030–1038
Halsey KH, Milligan AJ, Behrenfeld MJ (2014) Contrasting strategies of photosynthetic energy utilization drive lifestyle strategies in ecologically important picoeukaryotes. Metabolites 4:260–280
Ho T-Y, Quigg AS, Finkel ZV, Milligan AJ, Wyman K, Falkowski PG, Morel FMM (2003) The elemental composition of some marine phytoplankton. J Phycol 39:1145–1159
Jakob T, Wagner H, Stehfest K, Wilhelm C (2007) A complete energy balance from photons to new biomass reveals a light- and nutrient-dependent variability in the metabolic costs of carbon assimilation. J Exp Bot 58(8):2101–2112
Kliphuis AMJ, Klok AJ, Martens DE, Lamers PP, Janssen M, Wijffels RH (2012) Metabolic modeling of Chlamydomonas reinhardtii: energy requirements for photoautotrophic growth and maintenance. J Appl Phycol 24(2):253–266
Kolber Z, Prasil O, Falkowski PG (1998) Measurements of variable fluorescence using fast repetition rate techniques: defining methodolgy and experimental protocols. Biochim Biophys Acta 1367:88–106
Kooistra WHCF, Medlin LK (2007) Species concepts in diatoms: a clarification. Diatom Res 22:227–228
Kunath C, Jakob T, Wilhelm C (2012) Different phycobilin antenna organisations affect the balance between light use and growth rate in the cyanobacterium Microcystis aeruginosa and in the cryptophyte Cryptomonas ovata. Photosynth Res 111(1–2):173–183
Langdon C (1988) On the causes of interspecific differences in the growth-irradiance relationship for phytoplankton. J Plankton Res 37:715–730
Laws EA, Bannister TT (1980) Nutrient- and light-limited growth of Thalassiosira fluviatilis in continuous culture, with implicaitons for phytoplankton growth in the ocean. Limnol Oceanogr 25(3):457–473
MacIntyre HL, Cullen JJ (2005) Using cultures to investigate the physiological ecology of microalgae. In: Andersen RA (ed) Algal culturing techniques. Elsevier, Amsterdam, pp 287–326
MacIntyre HL, Kana TM, Anning T, Geider RJ (2002) Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. J Phycol 38:17–38
Margalef R (1978) Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol Acta 1:493–509
Mitchell BG, Kahru M, Wieland J, Stramska M (2003) Determination of spectral absorption coefficients of particles, dissolved material and phytoplankton for discrete water samples. In: Mueller JL, Fargoin GS, McClain CR (eds) Ocean optics protocols for satellite ocean color sensor validation, revision 4, vol 4. NASA/TM-2003, Greenbelt
Morris I (1980) Paths of carbon assimilation in marine phytoplankton. In: Falkowski PG (ed) Primary productivity in the sea. Plenum Press, New York, pp 139–159
Oxborough K, Moore CM, Suggett D, Lawson T, Chan HG, Geider RJ (2012) Direct estimation of functional PSII reaction center concentration and PSII electron flux on a volume basis: a new approach to the analysis of Fast Repetition Rate fluorometry (FRRf) data. Limnol Oceanogr Methods 10(3):142–154
Post AF, Dubinsky Z, Wyman K, Falkowski PG (1985) Physiological responses of a marine planktonic diatom to transitions in growth irradiance. Mar Ecol Prog Ser 25:141–149
Prihoda J, Tanaka A, de Paula WBM, Allen JF, Tirichine L, Bowler C (2012) Chloroplast-mitochondria cross-talk in diatoms. J Exp Bot 63(4):1543–1557
Raven JA (1984) A cost-benefit analysis of photon absorption by photosynthetic unicells. New Phytol 98:593–625
Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41
Sarthou G, Timmermans KR, Blain S, Tréguer P (2005) Growth physiology and fate of diatoms in the ocean: a review. J Sea Res 53:25–42
Silsbe GM, Oxborough K, Suggett DJ, Forster RM, Ihnken S, Komárek O, Lawrenz E, Prášil O, Röttgers R, Šicner M, Simis S, Van Dijk MA, Kromkamp JC (2015) Toward autonomous measurements of photsynthetic electron transport rates: an evaluation of active fluorescence-based measurements of photochemistry. Limnol Oceanogr Methods 13:138–155
Singh D, Carlson R, Fell D, Poolman M (2015) Modelling metabolism of the diatom Phaeodactylum tricornutum. Biochem Soc Trans 43(6):1182–1186
Six C, Finkel ZV, Rodriguez F, Marie D, Partensky F, Campbell DA (2008) Contrasting photoacclimation costs in ecotypes of the marine eukaryotic picoplankter Ostreococcus. Limnol Oceanogr 53(1):255–265
Smetacek V (1999) Diatoms and the ocean carbon cycle. Protist 150:25–32
Smith REH, Geider RJ (1985) Kinetics of intracellular carbon allocation in a marine diatom. J Exp Mar Biol Ecol 93:191–210
Smith REH, Platt T (1984) Carbon exchange and 14C tracer methods in a nitrogen-limited diatom Thalassiosira pseudonana. Mar Ecol Prog Ser 16(1–2):75–87
Smith SR, Abbriano RM, Hildebrand M (2012) Comparative analysis of diatom genomes reveals substantial differences in the organization of carbon partitioning pathways. Algal Res 1(1):2–16
Su W, Jakob T, Wilhelm C (2012) The impact of nonphotochemical quenching of fluorescence on the photon balance in diatoms under dynamic light conditions. J Phycol 48:336–346
Suggett DJ, Moore CM, Hickman AE, Geider RJ (2009) Interpretation of fast repetition rate (FRR) fluorescence: signatures of phytoplankton community structure versus physiological state. Mar Ecol Prog Ser 376:1–19
Sukenik A, Livne A (1991) Variations in lipid and faty-acid content in relation to acetyl CoA carboxylase in the marine prymnesiophyte Isochrysis-galbana. Plant Cell Physiol 32(3):371–378
Thamatrakoln K, Bailleul B, Brown CM, Gorbunov MY, Kustka AB, Frada M, Joliot PA, Falkowski PG, Bidle KD (2013) Death-specific protein in a marine diatom regulates photosynthetic responses to iron and light availability. Proc Natl Acad Sci 110(50):20123–20128
Vandenhecke JMR, Bastedo J, Cockshutt AM, Campbell DA, Huot Y (2015) Changes in the Rubisco to photosystem ratio dominate photoacclimation across phytoplankton taxa. Photosynth Res 124(3):275–291
Wagner H, Jakob T, Wilhelm C (2006) Balancing the energy flow from captured light to biomass under fluctuating light conditions. New Phytol 169:95–108
Weger HG, Herzig R, Falkowski PG, Turpin DH (1989) Respiratory losses in the light in a marine diatom: measurements by short-term mass spectrometry. Limnol Oceanogr 34(7):1153–1161
Wilhelm C, Buchel C, Fisahn J, Goss R, Jakob T, LaRoche J, Lavaud J, Lohr M, Riebesell U, Stehfest K, Valentin K, Kroth PG (2006) The regulation of carbon and nutrient assimilation in diatoms is significantly different from green algae. Protist 157:91–124
Acknowledgments
The authors thank Dr. Douglas Campbell and an anonymous reviewer for their careful reading and comments and suggestions that significantly improved the final manuscript. We also thank Greg Silsbe for helpful discussions during the final stages of manuscript preparation. This work was funded by a grant from the National Science Foundation Biological Oceanography program (NSF-OCE 1057244).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fisher, N.L., Halsey, K.H. Mechanisms that increase the growth efficiency of diatoms in low light. Photosynth Res 129, 183–197 (2016). https://doi.org/10.1007/s11120-016-0282-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-016-0282-6