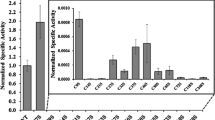
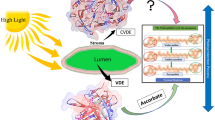
Abstract
Violaxanthin de-epoxidase (VDE) is a conditionally soluble enzyme located in the thylakoid lumen and catalyses the conversion of violaxanthin to antheraxanthin and zeaxanthin, which are located in the thylakoid membrane. These reactions occur when the plant or algae are exposed to saturating light and the zeaxanthin formed is involved in the process of non-photochemical quenching that protects the photosynthetic machinery during stress. Oversaturation by light results in a reduction of the pH inside the thylakoids, which in turn activates VDE and the de-epoxidation of violaxanthin. To elucidate the structural events responsible for the pH-dependent activation of VDE, full length and truncated forms of VDE were studied at different pH using circular dichroism (CD) spectroscopy, crosslinking and small angle X-ray scattering (SAXS). CD spectroscopy showed the formation of α-helical coiled-coil structure, localised in the C-terminal domain. Chemical crosslinking of VDE showed that oligomers were formed at low pH, and suggested that the position of the N-terminal domain is located near the opening of lipocalin-like barrel, where violaxanthin has been predicted to bind. SAXS was used to generate models of monomeric VDE at high pH and also a presumably dimeric structure of VDE at low pH. For the dimer, the best fit suggests that the interaction is dominated by one of the domains, preferably the C-terminal domain due to the lost ability to oligomerise at low pH, shown in earlier studies, and the predicted formation of coiled-coil structure.






Similar content being viewed by others
References
Alfadhli A, Steel E, Finlay L, Bächinger HP, Barklis E (2002) Hantavirus nucleocapsid protein coiled-coil domains. J Biol Chem 277:27103–27108. doi:10.1074/jbc.M203395200
Arnoux P, Morosinotto T, Saga G, Bassi R, Pignol D (2009) A structural basis for the pH-dependent xanthophyll cycle in Arabidopsis thaliana. Plant Cell 21:2036–2044. doi:10.1105/tpc.109.068007
Arvidsson PO, Bratt CE, Carlsson M, Åkerlund HE (1996) Purification and identification of the violaxanthin deepoxidase as a 43 kDa protein. Photosynth Res 49:119–129. doi:10.1007/BF00117662
Barber J, Andersson B (1992) Too much of a good thing: light can be bad for photosynthesis. Trends Biochem Sci 17:61–66. doi:10.1016/0968-0004(92)90503-2
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Bratt CE, Arvidsson PO, Carlsson M, Åkerlund HE (1995) Regulation of violaxanthin de-epoxidase activity by pH and ascorbate concentration. Photosynth Res 45:169–175. doi:10.1007/BF00032588
Demmig-Adams B (1990) Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020(1):1–24. doi:10.1016/0005-2728(90)90088-L
Drozdetskiy A, Cole C, Procter J, Barton GJ (2015) JPred4: a protein secondary structure prediction server. Nucleic Acids Res. doi:10.1093/nar/gkv332
Eskling M, Arvidsson P-O, Åkerlund HE (1997) The xanthophyll cycle, its regulation and components. Physiol Plant 100:806–816. doi:10.1034/j.1399-3054.1997.1000407.x
Fufezan C, Simionato D, Morosinotto T (2012) Identification of key residues for pH dependent activation of violaxanthin de-epoxidase from Arabidopsis thaliana. PLoS One 7(4):e35669. doi:10.1371/journal.pone.0035669
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy Server. In: Walker John M (ed) The proteomics protocols handbook. Humana Press, New York, pp 571–607
Gisselsson A, Szilagyi A, Åkerlund HE (2004) Role of histidines in the binding of violaxanthin de-epoxidase to the thylakoid membrane as studied by site-directed mutagenesis. Physiol Plant 122:337–343. doi:10.1111/j.1399-3054.2004.00415.x
Greenfield N (1999) Applications of circular dichroism in protein and peptide analysis. Trends Anal Chem 18(4):236–244. doi:10.1016/S0165-9936(98)00112-5
Greenfield NJ (2006) Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc 1(6):2876–2890. doi:10.1038/nprot.2006.202
Hallin EI, Guo K, Åkerlund HE (2015) Violaxanthin de-epoxidase disulphides and their role in activity and thermal stability. Photosynth Res 124(2):191–198. doi:10.1007/s11120-015-0118-9
Hallin EI, Guo K, Åkerlund HE (2016) Functional and structural characterisation of domain truncated Violaxanthin de-epoxidase. Physiol Plant Accept. doi:10.1111/ppl.12428
Hieber D, Bugos R, Verhoeven A, Yamamoto H (2002) Overexpression of violaxanthin de-epoxidase: properties of C-terminal deletions on activity and pH-dependent lipid binding. Planta 214(3):476–483. doi:10.1007/s00425-001-0704-2
Kammerer RA, Schulthess T, Landwehr R, Lustig A, Engel J, Aebi U, Steinmetz MO (1998) An autonomous folding unit mediates the assembly of two-stranded coiled coils. PNAS 95(23):13419–13424. doi:10.1073/pnas.95.23.13419
Konarev PV, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI (2003) PRIMUS: a windows PC-based system for small-angle scattering data analysis. J Appl Crystallogr 36:1277–1282. doi:10.1107/S0021889803012779
Kramer DM, Sacksteder CA, Cruz JA (1999) How acidic is the lumen? Photosynth Res 60(2):151–163. doi:10.1023/A:1006212014787
Labrador A, Cerenius Y, Svensson C, Theodor K, Plivelic T (2013) The yellow mini-hutch for SAXS experiments at MAX IV Laboratory. J Phys Conf Ser 425:072019. doi:10.1088/1742-6596/425/7/072019
Latowski D, Åkerlund HE, Strzałka K (2004) Violaxanthin de-epoxidase, the xanthophyll cycle enzyme, requires lipid inverted hexagonal structures for its activity. Biochemistry 43(15):4417–4420. doi:10.1021/bi049652g
Lupas AN, Gruber M (2005) The structure of alpha-helical coiled coils. Adv Protein Chem 70:37–78. doi:10.1016/S0065-3233(05)70003-6
Muller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566. doi:10.1104/pp.125.4.1558
Petoukhov MV, Franke D, Shkumatov AV, Tria G, Kikhney AG, Gajda M, Gorba C, Mertens HDT, Konarev PV, Svergun DI (2012) New developments in the ATSAS program package for small-angle scattering data analysis. J Appl Crystallogr 45(2):342–350. doi:10.1107/S0021889812007662
Saga G, Giorgetti A, Christian Fufezan C, Giacometti GM, Bassi R, Morosinotto T (2010) Mutation analysis of violaxanthin de-epoxidase identifies substrate-binding sites and residues involved in catalysis. J Biol Chem 285:23763–23770. doi:10.1074/jbc.M110.115097
Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1:2856–2860. doi:10.1038/nprot.2006.468
Simionato D, Basso S, Zaffagnini M, Lana T, Marzotto F, Trost P, Morosinotto T (2015) Protein redox regulation in the thylakoid lumen: the importance of disulfide bonds for violaxanthin de-epoxidase. FEBS Lett 589(8):919–923. doi:10.1016/j.febslet.2015.02.033
Steinmetz MO, Jelesarov I, Matousek WM, Honnappa S, Jahnke W, Missimer JH, Frank S, Alexandrescu AT, Kammerer RA (2007) Molecular basis of coiled-coil formation. PNAS 104(17):7062–7067. doi:10.1073/pnas.0700321104
Svergun DI (1992) Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J Appl Crystallogr 25:495–503. doi:10.1107/S0021889892001663
Svergun DI (1999) Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys J 76(6):2879–2886. doi:10.1016/S0006-3495(99)77443-6
Svergun DI, Petoukhov MV, Koch MH (2001) Determination of domain structure of proteins from X-ray solution scattering. Biophys J 80(6):2946–2953. doi:10.1016/S0006-3495(01)76260-1
Szilágyi A, Sommarin M, Åkerlund HE (2007) Membrane curvature stress controls the maximal conversion of violaxanthin to zeaxanthin in the violaxanthin cycle—influence of α-tocopherol, cetylethers, linolenic acid, and temperature. Biochim Biophys Acta 1768(9):2310–2318. doi:10.1016/j.bbamem.2007.06.001
Szilágyi A, Selstam E, Åkerlund HE (2008) Laurdan fluorescence spectroscopy in the thylakoid bilayer: the effect of violaxanthin to zeaxanthin conversion on the galactolipid dominated lipid environment. Biochim Biophys Acta 1778(1):348–355. doi:10.1016/j.bbamem.2007.10.006
Tamoi M, Nagaoka M, Miyagawa Y, Shigeoka S (2006) Contribution of fructose-1,6-bisphosphatase and Sedoheptulose-1,7-bisphosphatase to the photosynthetic rate and carbon flow in the calvin cycle in transgenic plants. Plant Cell Physiology 47(3):380–390. doi:10.1093/pcp/pcj004
Volkov VV, Svergun DI (2003) Uniqueness of ab initio shape determination in small-angle scattering. J Appl Crystallogr 36:860–864. doi:10.1107/S0021889803000268
Acknowledgments
This work was financially supported by Carl Trygger Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hallin, E.I., Hasan, M., Guo, K. et al. Molecular studies on structural changes and oligomerisation of violaxanthin de-epoxidase associated with the pH-dependent activation. Photosynth Res 129, 29–41 (2016). https://doi.org/10.1007/s11120-016-0261-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-016-0261-y