Abstract
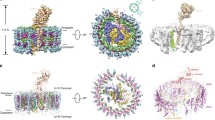
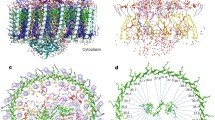
The photosynthetic membranes of the filamentous anoxygenic phototroph Roseiflexus castenholzii have been studied with electron microscopy, atomic force microscopy, and biochemistry. Electron microscopy of the light-harvesting reaction center complex produced a 3D model that aligns with the solved crystal structure of the RC–LH1 from Thermochromatium tepidum with the H subunit removed. Atomic force microscopy of the whole membranes yielded a picture of the supramolecular organization of the major proteins in the photosynthetic electron transport chain. The results point to a loosely packed membrane without accessory antenna proteins or higher order structure.







Similar content being viewed by others
Abbreviations
- ACIII:
-
Alternative complex III
- AFM:
-
Atomic force microscopy
- Bchl:
-
Bacteriochlorophyll
- Bphe:
-
Bacteriopheophytin
- EM:
-
Electron microscopy
- FAP:
-
Filamentous anoxygenic phototrophs
- GSB:
-
Green sulfur bacteria
- RC–LH:
-
Light-harvesting reaction center complex in Roseiflexus castenholzii
- RC–LH1:
-
Light-harvesting reaction center 1 complex in purple bacteria
References
Adams PG, Hunter CN (2012) Adaptation of intracytoplasmic membranes to altered light intensity in Rhodobacter sphaeroides. Biochim Biophys Acta 1817:1616–1627
Allen JP, Feher G, Yeates TO et al (1987) Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc Natl Acad Sci USA 84:6162–6166
Bahatyrova S, Frese RNR, Siebert CA et al (2004) The native architecture of a photosynthetic membrane. Nature 430:1058–1062
Bína D, Gardian Z, Vácha F, Litvín R (2014) Supramolecular organization of photosynthetic membrane proteins in the chlorosome-containing bacterium Chloroflexus aurantiacus. Photosynth Res 122:13–21
Blankenship RE (2010) Early evolution of photosynthesis. Plant Physiol 154:434–438
Boonstra AF, Visschers RW, Calkoen F et al (1993) Structural characterization of the B800–850 and B875 light-harvesting antenna complexes from Rhodobacter sphaeroides by electron microscopy. Biochim Biophys Acta 1142:181–188
Boonstra AF, Germeroth L, Boekema EJ (1994) Structure of the light harvesting antenna from Rhodospirillum molischianum studied by electron microscopy. Biochim Biophys Acta 1184:227–234
Broglie RM, Hunter CN, Delepelaire P et al (1980) Isolation and characterization of the pigment-protein complexes of Rhodopseudomonas sphaeroides by lithium dodecyl sulfate/polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA 77:87–91
Cao L, Bryant DA, Schepmoes AA et al (2012) Comparison of Chloroflexus aurantiacus strain J-10-fl proteomes of cells grown chemoheterotrophically and photoheterotrophically. Photosynth Res 110:153–168
Cartron ML, Olsen JD, Sener M et al (2014) Integration of energy and electron transfer processes in the photosynthetic membrane of Rhodobacter sphaeroides. Biochim Biophys Acta 1837:1369–1380
Cogdell R, van Grondelle R (2003) The light-harvesting system of purple bacteria. In: Green BR, Parson WW (eds) Light-harvesting antennas in photosynthesis. Advances in Photosynthesis, vol 13. Kluwer Academic Publishers, Dordrecht, pp 169–194
Collins AM, Xin Y, Blankenship RE (2009) Pigment organization in the photosynthetic apparatus of Roseiflexus castenholzii. Biochim Biophys Acta 1787:1050–1056
Collins A, Qian P, Tang Q, Bocian D (2010) Light-harvesting antenna system from the phototrophic bacterium Roseiflexus castenholzii. Biochemistry 49:7524–7531
Collins A, Kirmaier C, Holten D, Blankenship RE (2011) Kinetics and energetics of electron transfer in reaction centers of the photosynthetic bacterium Roseiflexus castenholzii. Biochim Biophys Acta 1807:262–269
Comayras F, Jungas C, Lavergne J (2005) Functional consequences of the organization of the photosynthetic apparatus in Rhodobacter sphaeroides. I. Quinone domains and excitation transfer in chromatophores and reaction center.antenna complexes. J Biol Chem 280:11203–11213
Crouch L, Jones M (2012) Cross-species investigation of the functions of the Rhodobacter PufX polypeptide and the composition of the RC–LH1 core complex. Biochim Biophys Acta 2:336–352
Deisenhofer J, Epp O, Miki K et al (1985) Structure of the protein subunits in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3 Å resolution. Nature 318:618–624
Gao X, Xin Y, Blankenship RE (2009) Enzymatic activity of the alternative complex III as a menaquinol:auracyanin oxidoreductase in the electron transfer chain of Chloroflexus aurantiacus. FEBS Lett 583:3275–3279
Gao X, Xin Y, Bell PD et al (2010) Structural analysis of alternative complex III in the photosynthetic electron transfer chain of Chloroflexus aurantiacus. Biochemistry 49:6670–6679
Gao X, Majumder EW, Kang Y et al (2013) Functional analysis and expression of the mono-heme containing cytochrome c subunit of alternative complex III in Chloroflexus aurantiacus. Arch Biochem Biophys 535:197–204
Goodhew CF, Brown KR, Pettigrew GW (1986) Haem staining in gels, a useful tool in the study of bacterial c-type cytochromes. Biochim Biophys Acta 852:288–294
Guner S, Willie A, Millett F et al (1993) The interaction between cytochrome c2 and the cytochrome bc1 complex in the photosynthetic purple bacteria Rhodobacter capsulatus and Rhodopseudomonas viridis. Biochemistry 32:4793–4800
Hall J, Zha X, Durham B et al (1987) Reaction of cytochrome c and c2 with the Rhodobacter sphaeroides reaction center involves the heme crevice domain. Biochemistry 26:4494–4500
Hanada S, Takaichi S, Matsuura K, Nakamura K (2002) Roseiflexus castenholzii gen. nov., sp nov., a thermophilic, filamentous, photosynthetic bacterium that lacks chlorosomes. Int J Syst Evol Microbiol 52:187–193
Hohmann-Marriott MF, Blankenship RE (2011) Evolution of photosynthesis. Annu Rev Plant Biol 62:515–548
Hu X, Ritz T (2002) Photosynthetic apparatus of purple bacteria. Q Rev Biophys 35:1–62
Jormakka M, Yokoyama K, Yano T et al (2008) Molecular mechanism of energy conservation in polysulfide respiration. Nat Struct Mol Biol 15:730–737
Katona G, Andréasson U, Landau EM et al (2003) Lipidic cubic phase crystal structure of the photosynthetic reaction centre from Rhodobacter sphaeroides at 2.35 Å resolution. J Mol Biol 331:681–692
Kirmaier C, Holten D (1987) Primary photochemistry of reaction centers from the photosynthetic purple bacteria. Photosynth Res 13:225–260
Kirmaier C, Holten D, Parson WW (1985) Temperature and detection-wavelength dependence of the picosecond electron-transfer kinetics measured in Rhodopseudomonas sphaeroides reaction centers. Resolution of new spectral and kinetic components in the primary charge-separation process. Biochim Biophys Acta 810:33–48
Liu H, Zhang H, Niedzwiedzki DM et al (2013) Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria. Science 342:1104–1107
Majumder ELW, King JD, Blankenship RE (2013) Alternative Complex III from phototrophic bacteria and its electron acceptor auracyanin. Biochim Biophys Acta 11:1383–1391
Niedzwiedzki DM, Collins AM, LaFountain AM et al (2010) Spectroscopic studies of carotenoid-to-bacteriochlorophyll energy transfer in RC-LH photosynthetic complex from Roseiflexus castenholzii. J Phys Chem B 114:8723–8734
Niwa S, Yu L, Takeda K et al (2014) Structure of the LH1-RC complex from Thermochromatium tepidum at 3.0 Å. Nature 508:228–232
Ortega JM, Mathis P (1993) Electron transfer from the tetraheme cytochrome to the special pair in isolated reaction centers of Rhodopseudomonas viridis. Biochemistry 32:1141–1151
Pettersen EF, Goddard TD, Huang CC et al (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Pierson BK, Castenholz RW (1974) A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol 100:5–24
Pugh R, McGlynn P, Jones M, Hunter C (1998) The LH1–RC core complex of Rhodobacter sphaeroides: interaction between components, time-dependent assembly, and topology of the PufX protein. Biochim Biophys Acta 1366:301–316
Pullerits T, Sundström V (1996) Photosynthetic light-harvesting pigment-protein complexes: toward understanding how and why. Acc Chem Res 8:381–389
Pullerits T, Visscher KJ, Hess S et al (1994) Energy transfer in the inhomogeneously broadened core antenna of purple bacteria: a simultaneous fit of low-intensity picosecond absorption and fluorescence kinetics. Biophys J 66:236–248
Qian P, Hunter C, Bullough P (2005) The 8.5 Å projection structure of the core RC–LH1–PufX dimer of Rhodobacter sphaeroides. J Mol Biol 349:948–960
Qian P, Bullough P, Hunter C (2008) Three-dimensional reconstruction of a membrane-bending complex the RC-LH1-PufX core dimer of Rhodobacter sphaeroides. J Biol Chem 283:14002–14011
Qian P, Papiz MZ, Jackson PJ et al (2013) Three-dimensional structure of the Rhodobacter sphaeroides RC-LH1-PufX complex: dimerization and quinone channels promoted by PufX. Biochemistry 43:7575–7585
Roszak AW, Howard TD, Southall J et al (2003) Crystal structure of the RC-LH1 core complex from Rhodopseudomonas palustris. Science 302:1969–1972
Scheuring S, Sturgis JN (2009) Atomic force microscopy of the bacterial photosynthetic apparatus: plain pictures of an elaborate machinery. Photosynth Res 102:197–211
Scheuring S, Lévy D, Rigaud J-L (2005) Watching the components of photosynthetic bacterial membranes and their in situ organisation by atomic force microscopy. Biochim Biophys Acta 1712:109–127
Scheuring S, Boudier T, Sturgis J (2007) From high-resolution AFM topographs to atomic models of supramolecular assemblies. J Struct Biol 159:268–276
Sener MK, Olsen JD, Hunter CN, Schulten K (2007) Atomic-level structural and functional model of a bacterial photosynthetic membrane vesicle. Proc Natl Acad Sci USA 104:15723–15728
Sturgis JNJ, Niederman RRA (2008) Atomic force microscopy reveals multiple patterns of antenna organization in purple bacteria: implications for energy transduction mechanisms and membrane modeling. Photosynth Res 95:269–278
Sturgis JN, Tucker JD, Olsen JD et al (2009) Atomic force microscopy studies of native photosynthetic membranes. Biochemistry 48:3679–3698
Sundström V, van Grondelle R (1995) Kinetics of excitation transfer and trapping in purple bacteria. Anoxyg Photosynth Bact 2:349–372
Takaichi S, Maoka T, Yamada M et al (2001) Absence of carotenes and presence of a tertiary methoxy group in a carotenoid from a thermophilic filamentous photosynthetic bacterium Roseiflexus castenholzii. Plant Cell Physiol 42:1355–1362
Tang G, Peng L, Baldwin PR et al (2007) EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol 157:38–46
Tang K-H, Barry K, Chertkov O et al (2011) Complete genome sequence of the filamentous anoxygenic phototrophic bacterium Chloroflexus aurantiacus. BMC Genomics 12:334
Timmins PA, Leonhard M, Weltzien HU et al (1988) A physical characterization of some detergents of potential use for membrane protein crystallization. FEBS Lett 238:361–368
Tsukatani Y, Nakayama N, Shimada K et al (2009) Characterization of a blue-copper protein, auracyanin, of the filamentous anoxygenic phototrophic bacterium Roseiflexus castenholzii. Arch Biochem Biophys 490:57–62
Tucker JD, Siebert CA, Escalante M et al (2010) Membrane invagination in Rhodobacter sphaeroides is initiated at curved regions of the cytoplasmic membrane, then forms both budded and fully detached spherical vesicles. Mol Microbiol 76:833–847
Van der Meer MTJ, Klatt CG, Wood J et al (2010) Cultivation and genomic, nutritional, and lipid biomarker characterization of Roseiflexus strains closely related to predominant in situ populations inhabiting Yellowstone hot spring microbial mats. J Bacteriol 192:3033–3042
Van ver Meer MTJ, Schouten S, Hanada S et al (2002) Alkane-1,2-diol-based glycosides and fatty glycosides and wax esters in Roseiflexus castenholzii and hot spring microbial mats. Arch Microbiol 178:229–237
Visscher KJ, Bergström H, Sundström V et al (1989) Temperature dependence of energy transfer from the long wavelength antenna BChl-896 to the reaction center in Rhodospirillum rubrum, Rhodobacter sphaeroides (w.t. and M21 mutant) from 77 to 177 K, studied by picosecond absorption spectroscopy. Photosynth Res 22:211–217
Xin Y, Pan J, Collins A et al (2012) Excitation energy transfer and trapping dynamics in the core complex of the filamentous photosynthetic bacterium Roseiflexus castenholzii. Photosynth Res 111:149–156
Yamada M, Zhang H, Hanada S et al (2005) Structural and spectroscopic properties of a reaction center complex from the chlorosome-lacking filamentous anoxygenic phototrophic bacterium Roseiflexus castenholzii. J Bacteriol 187:1702–1709
Acknowledgments
This material is based upon work supported as part of the Photosynthetic Antenna Research Center (PARC), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC 0001035; EL-WM was supported by an Olin Fellowship for Women and a PEO Scholar Award.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Majumder, E.LW., Olsen, J.D., Qian, P. et al. Supramolecular organization of photosynthetic complexes in membranes of Roseiflexus castenholzii . Photosynth Res 127, 117–130 (2016). https://doi.org/10.1007/s11120-015-0179-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0179-9