Abstract
Effects of ocean acidification on Emiliania huxleyi strain RCC 1216 (calcifying, diploid life-cycle stage) and RCC 1217 (non-calcifying, haploid life-cycle stage) were investigated by measuring growth, elemental composition, and production rates under different pCO2 levels (380 and 950 μatm). In these differently acclimated cells, the photosynthetic carbon source was assessed by a 14C disequilibrium assay, conducted over a range of ecologically relevant pH values (7.9–8.7). In agreement with previous studies, we observed decreased calcification and stimulated biomass production in diploid cells under high pCO2, but no CO2-dependent changes in biomass production for haploid cells. In both life-cycle stages, the relative contributions of CO2 and HCO3 − uptake depended strongly on the assay pH. At pH values ≤ 8.1, cells preferentially used CO2 (≥ 90 % CO2), whereas at pH values ≥ 8.3, cells progressively increased the fraction of HCO3 − uptake (~45 % CO2 at pH 8.7 in diploid cells; ~55 % CO2 at pH 8.5 in haploid cells). In contrast to the short-term effect of the assay pH, the pCO2 acclimation history had no significant effect on the carbon uptake behavior. A numerical sensitivity study confirmed that the pH-modification in the 14C disequilibrium method yields reliable results, provided that model parameters (e.g., pH, temperature) are kept within typical measurement uncertainties. Our results demonstrate a high plasticity of E. huxleyi to rapidly adjust carbon acquisition to the external carbon supply and/or pH, and provide an explanation for the paradoxical observation of high CO2 sensitivity despite the apparently high HCO3 − usage seen in previous studies.
Similar content being viewed by others
Introduction
Marine phytoplankton account for ~50 % of global primary production and are the main drivers of the marine "particulate organic carbon" (POC) pump (Falkowski et al. 1998; Field et al. 1998). Calcifying phytoplankton species also contribute to the "particulate inorganic carbon" (PIC) pump and thereby play a dual role in regulating marine biogeochemical cycling of carbon through their effects on surface ocean alkalinity (Broecker and Peng 1982; Zeebe and Wolf-Gladrow 2007). One key species of calcifying phytoplankton is the cosmopolitan and bloom-forming coccolithophore Emiliania huxleyi, which has been established as a model organism over the recent decades (Paasche 2002; Raven and Crawfurd 2012; Read et al. 2013; Westbroek et al. 1993). While the calcifying diploid life-cycle stage of this species has been intensively studied in field and laboratory experiments, the non-calcifying haploid stage has only recently gained attention due to its important ecological role. In blooms of diploid E. huxleyi, which are usually terminated by viruses, the haploid life-cycle stage functions as a virus-resistant backup population (Frada et al. 2012). Furthermore, the presence and absence of calcification in the differing life-cycle stages of E. huxleyi make them ideal candidates to investigate the cellular mechanisms of calcification and their interaction with photosynthesis under increasing oceanic CO2 concentrations (Mackinder et al. 2010; Rokitta and Rost 2012).
Increasing pCO2 in oceanic surface water directly affects carbonate chemistry by elevating the concentration of dissolved inorganic carbon (DIC) and shifting the carbon speciation toward higher CO2 and H+ concentrations, a phenomenon often referred to as ocean acidification (OA; Caldeira and Wickett 2003; Wolf-Gladrow et al. 1999). Compared to preindustrial values, pH is expected to drop by 0.4–0.5 units until the end of this century. In several studies testing the effects of OA on E. huxleyi, diploid strains were found to exhibit strong, yet opposing responses in terms of biomass and calcite production. While biomass production was either unaffected or stimulated by increased pCO2, calcification typically decreased and malformations of coccoliths increased (e.g., Hoppe et al. 2011; Langer et al. 2009; Riebesell et al. 2000). Bach et al. (2011) suggested that biomass production is stimulated by increasing CO2 concentration at sub-saturating conditions, whereas calcification is specifically responsive to the associated decrease in pH. Such differential CO2 and pH effects on biomass and calcite production are supported by the observation that OA distorts ion homeostasis and shifts the metabolism from oxidative to reductive pathways (Rokitta et al. 2012; Taylor et al. 2011). In a number of studies, the sensitivity of E. huxleyi toward OA has been attributed to its mode of inorganic carbon (Ci) acquisition, which is intrinsically responsive to changes in carbonate chemistry. Thus, for understanding the differential responses to OA, one needs to look at this crucial process of Ci assimilation.
Like most phytoplankton, E. huxleyi operates a CO2 concentrating mechanism (CCM), which utilizes CO2 and/or HCO3 − uptake systems to accumulate CO2 in the vicinity of RubisCO, and employs the enzyme carbonic anhydrase (CA) to accelerate the inter-conversion between these Ci species (see Reinfelder 2011 for review). For a long time, the CCM in E. huxleyi was assumed to rely on the CO2 delivery by calcification (Anning et al. 1996; Sikes et al. 1980). More recently, however, studies have demonstrated that Ci fluxes for photosynthesis and calcification are independent (Herfort et al. 2004; Rost et al. 2002; Trimborn et al. 2007), and that these two processes may even compete for Ci substrates (Rokitta and Rost 2012). Most studies performed on the CCM of E. huxleyi to date yielded moderately high substrate affinities for Ci, which decreased slightly under OA scenarios (e.g., Rokitta and Rost 2012; Rost et al. 2003, Stojkovic et al. 2013). Moreover, low activity for extracellular CA and high contribution of HCO3 − uptake for photosynthesis have been reported (e.g., Herfort et al. 2002; Rokitta and Rost 2012; Stojkovic et al. 2013; Trimborn et al. 2007). This high apparent HCO3 − usage is puzzling, however, as it suggests biomass production to be rather insensitive to OA-related changes in CO2 supply, which is in contrast to what studies usually have observed.
Most physiological methods characterizing the CCM and its functional elements are performed under standardized assay conditions, including a fixed pH value, and thus differing from treatment conditions. The pH and the concominant Ci speciation can, however, influence the cell’s physiology, in particular its Ci acquisition. When identifying the cause-effect relationship in OA responses, it is difficult to separate the effects of changes in Ci speciation from concomitant changes in H+ concentrations. Changes in external pH have been shown to directly drive changes in cytosolic pH in E. huxleyi, which, in turn, affected H+ gradients and membrane potentials (Suffrian et al. 2011; Taylor et al. 2011). This effect could indirectly impact secondary active transporters, e.g., the Cl−/HCO3 − antiporter (Herfort et al. 2002; Rokitta et al. 2011). Moreover, the protonation of amino acid side chains can affect activity, specificity, and kinetics of enzymes and transporters involved in cellular processes (Badger 2003; Raven 2006). Hence, aside from altered concentrations of Ci species, pH itself could directly impact the mode of CCM (Raven 1990). These possible effects of the assay pH on Ci acquisition should be accounted for when performing experiments to characterize the CCM.
One common approach to determine the Ci source for photosynthesis is the application of the 14C disequilibrium method (Espie and Colman 1986), which has proven suitable for the study of marine phytoplankton in laboratory cultures (e.g., Elzenga et al. 2000; Rost et al. 2006a) and in natural field assemblages (e.g., Cassar et al. 2004; Martin and Tortell 2006; Tortell and Morel 2002; Tortell et al. 2008). The method makes use of the relatively slow chemical conversion between the CO2 and HCO3 − in the absence of CA (Johnson 1982), allowing for a differential labeling of these Ci species with 14C. This method is typically performed at pH of 8.5 ("assay pH"), deviating strongly from most natural in situ values and even more from the pH values applied in OA-experiments ("acclimation pH"). In this study, we aimed to disentangle the short-term effect of assay pH from the long-term effect of acclimation history on the photosynthetic Ci source of E. huxleyi. To this end, we grew haploid and diploid life-cycle stages at present-day (380 μatm) and elevated pCO2 (950 μatm), and measured the responses in growth, elemental composition, and production rates. These low and high pCO2-acclimated cells were then tested for their preferred Ci source by applying the 14C disequilibrium method, with assay conditions set to a range of ecologically relevant pH values (pH 7.9–8.7). The reliability of this new approach was tested by performing sensitivity studies.
Methods
pCO2 acclimations
Haploid and diploid cells of E. huxleyi (strains RCC 1217 and RCC 1216, obtained from the Roscoff culture collection) were grown at 15 °C as dilute batch incubations. North Sea seawater medium (salinity 32.4) was sterile-filtered (0.2 μm) and enriched with vitamins and trace metals according to F/2 (Guillard and Ryther 1962), as well as phosphate and nitrate (100 and 6.25 μmol L−1). Cells were exposed to a light:dark cycle (16:8 h) and saturating light (300 μmol photons m−2 s−1) provided by daylight lamps (FQ 54W/965HO, OSRAM, Munich, Germany). Light intensity was monitored with the LI-6252 datalogger (LI-COR, Lincoln, NE, USA) using a 4π-sensor (US-SQS/L, Walz, Effeltrich, Germany). Culturing was carried out in sterilized 2.4 L borosilicate bottles (Duran Group, Mainz, Germany) on roller tables to avoid sedimentation.
Prior to experiments, cells were acclimated to the respective pCO2 and light conditions for at least 7 days (i.e., more than 10 generations). Prior to initiating cultures, medium was pre-aerated for at least 36 h with humidified, 0.2 μm-filtered air comprising pCO2 values of 380 or 950 μatm (equivalent to 38.5 and 96.3 Pa, or ~15 and ~35 μmol kg−1, respectively). Gas mixtures were created by a gas flow controller (CGM 2000 MCZ Umwelttechnik, Bad Nauheim, Germany) using pure CO2 (Air Liquide Deutschland, Düsseldorf, Germany) and CO2-free air (CO2RP280, Dominick Hunter, Willich, Germany). Sampling and measurements were done 4–8 h after the beginning of the light period (i.e., at midday) in exponential growth at densities of 40,000–60,000 cells mL−1. Cultures showing a pH drift of > 0.05 were excluded from further analyses.
The carbonate system (Table 1) during the acclimations was assessed based on measurements of pH and total alkalinity (TA). The pHNBS of the cultures was measured potentiometrically and corrected for temperature (pH-meter 3110; WTW, Weilheim, Germany). The electrode (A157, Schott Instruments, Mainz, Germany) was three-point calibrated with NBS certified standard buffers and the measurement uncertainty was 0.03 pH units. TA was determined by potentiometric titration (Dickson 1981; TitroLine alpha plus, Schott Instruments). Measurements were accuracy-corrected with certified reference materials (CRMs) supplied by A. Dickson (Scripps Institution of Oceanography, USA). Calculation of the carbonate system was performed using CO2sys (Pierrot et al. 2006). Input parameters were pHNBS and TA, as well as temperature (15 °C), salinity (32.4), and pressure (1 dbar, according to 1 m depth; Hoppe et al. 2012). For all calculations, phosphate and silicate concentrations were assumed to be 7 and 17 μmol kg−1, respectively, based on assessments of the media. Equilibrium constants for carbonic acid, K1 and K2 given by Mehrbach et al. (1973) and refit by Dickson and Millero (1987) were used. For the dissociation of sulfuric acid, the constants reported by Dickson (1990) were employed.
Cell growth was assessed by daily cell counting with a Multisizer III hemocytometer (Beckman-Coulter, Fullerton, CA, USA) and the specific growth rates (μ) were calculated from daily increments (cf., Rokitta and Rost 2012). For the determination of total particulate carbon (TPC), POC and particulate organic nitrogen (PON), cell suspensions were vacuum-filtered (-200 mbar relative to atmosphere) onto pre-combusted (12 h, 500 °C) GF/F filters (1.2 μm; Whatman, Maidstone, UK), which were dried at 65 °C and analyzed with a EuroVector CHNS-O elemental analyzer (EuroEA, Milano, Italy). Before quantification of POC, filters were HCl-soaked (200 μL, 0.2 M) and dried to remove calcite. PIC was assessed as the difference between TPC and POC. By multiplying the POC and PIC cell quotas with μ, the respective production rates were derived (cf., Rokitta and Rost 2012). For Chl a measurements, cells were filtered onto cellulose nitrate filters (0.45 μm; Sartorius, Göttingen, Germany) and instantly frozen in liquid nitrogen. Chl a was extracted in 90 % acetone (v/v, Sigma, Munich, Germany) and determined fluorometrically (TD-700 fluorometer, Turner Designs, Sunnyvale, USA) following the protocol by Holm-Hansen and Riemann (1978). The calibration of the fluorometer was carried out with a commercially available Chl a standard (Anacystis nidulans, Sigma, Steinheim, Germany).
14C disequilibrium method
The Ci source for photosynthesis was determined by applying the 14C disequilibrium method (Elzenga et al. 2000; Espie and Colman 1986; Tortell and Morel 2002). In this method, a transient isotopic disequilibrium is induced by adding a small volume of a 14Ci "spike" solution with a relatively low pH (typically 7.0) into larger volume of buffered cell suspension with a relatively high pH (typically 8.5). The cell suspension contains dextran-bound sulfonamide (DBS) to eliminate possible external CA activity. Due to the pH-dependent speciation of DIC, the relative CO2 concentration of the spike is high (~19 % of DIC at pH 7.0), compared to the cell suspension (~0.3 % of DIC at pH 8.5). When adding the spike to the cell suspension, the majority of the CO2 added with the spike converts into HCO3 − until equilibrium is achieved (Johnson 1982; Millero and Roy 1997). Consequently, the specific activity of CO2 (\({\text{SA}}_{{{\text{CO}}_{2} }}\), dpm (mol CO2)−1) is initially high and exponentially decays over time (Fig. 1). The slope of the 14C incorporation curve of a "CO2 user" is, therefore, initially much steeper than during final linear 14C uptake, when isotopic equilibrium is achieved. In contrast, the slope of 14C incorporation for "HCO3 − users" changes only marginally over time because \({\text{SA}}_{{{\text{HCO}}_{3}^{ - } }}\) stays more or less constant during the assay.
Time-course of specific activities of CO2 and HCO3 − (medium and long dashed lines, respectively, here calculated for assay pH 8.5) in the isotopic disequilibrium method and examples for the 14C incorporation of the diploid life-cycle stage for predominant CO2 usage (\(f_{{{\text{CO}}_{ 2} }} = 1.00\), squares) and considerable HCO3 − usage (\(f_{{{\text{CO}}_{ 2} }} = 0.60\), triangles)
Quantification of the relative proportion of CO2 or HCO3 − usage was done by fitting data with the integral function of the 14C fixation rate (Elzenga et al. 2000; Espie and Colman 1986; Martin and Tortell 2006). The function includes terms representing the instantaneous fixation rate of DI14C, the fractional contribution of CO2 \(\left( {f_{{{\text{CO}}_{2} }} } \right)\) or HCO3 − usage \(\left( {1 - f_{{{\text{CO}}_{2} }} } \right)\) to the overall Ci fixation and the specific activity (SA, dpm mol−1) of these substrates at any given time (Eq. 1; Espie and Colman 1986; Elzenga et al. 2000; Tortell and Morel 2002). Strictly speaking, as HCO3 − and CO3 2− cannot be differentially labeled, \(1 - f_{{{\text{CO}}_{2} }}\) also comprises the potential fraction of CO3 2− used.
In this equation, V DI14C is the total rate of 14C uptake; \(f_{{{\text{CO}}_{ 2} }}\) is the fraction of uptake attributable to CO2; α 1 and α 2 are the temperature-, salinity-, and pH-dependent first-order rate constants for CO2 and HCO3 − hydration and dehydration, respectively; t is the time (s); \(\Delta {\text{SA}}_{{{\text{CO}}_{2} }}\) and \(\Delta {\text{SA}}_{{{\text{HCO}}_{3} }}\) are the differences between the initial and equilibrium values of the specific activities of CO2 and HCO3 −, respectively; and SADIC is the specific activity of DIC. During steady-state photosynthesis, VDI14C and \(f_{{{\text{CO}}_{ 2} }}\) are assumed to be constant so that changes in the instantaneous 14C uptake rate reflect only changes in the specific activity of CO2 and HCO3 −.
In the present study, the 14C disequilibrium method was modified to enable measurements over a range of ecologically relevant pH values (7.90–8.70). In order to maintain a suitably large initial isotopic disequilibrium \(\left( {\Delta {\text{SA}}_{{{\text{CO}}_{2} }} / {\text{SA}}_{\text{DIC}} } \right)\), the pH of the 14C spike solutions needs to be adjusted in conjunction with the pH of the assay buffer. We, thus, used either MES or HEPES buffers to set the pH of spike solutions over the range of 5.75–7.30 (see Table 2 for exact pH values of assay and spike buffers). For the assays, 10–30 × 106 cells were concentrated via gentle filtration over a polycarbonate filter (2 μm; Millipore, Billerica, MA, USA) to a final volume of 15 mL. During this filtration procedure, cells were kept in suspension, while the medium was gradually exchanged with buffered assay medium of the appropriate pH value. Assay media and spike buffers were prepared at least 1 day prior to the assay and stored in closed containers to avoid CO2 exchange and pH drift. The pH value and temperatures of all buffers were measured immediately prior to assay runs. DIC concentration of the assay buffers was determined colorimetrically according to Stoll et al. (2001) using a TRAACS CS800 autoanalyzer (Seal Analytical, Norderstedt, Germany), and measurements were accuracy-corrected with CRMs supplied by A. Dickson (Scripps Institution of Oceanography, USA).
To initiate the assays, a volume of 4 mL buffered concentrated cell suspension was transferred into a temperature-controlled, illuminated glass cuvette (15 °C; 300 μmol photons m−2 s−1) to which 50 μM DBS was added (Ramidus, Lund, Sweden). Cells were continuously stirred in the light for at least 5 min prior to spike addition to reach steady-state photosynthesis. Spike solutions were prepared by adding NaH14CO3 solution (1.88 GBq (mmol DIC)−1; GE Healthcare, Amersham, UK) into a final volume of 200 μL of pH-buffered MilliQ water (various buffers at 20 mM; Table 2), yielding activities of ~370 kBq (10 μCi). Following the spike addition, 200 μL subsamples of the cell suspension were transferred into 2 mL HCl (6 M) at time points between 5 s and 12 min. Addition of these aliquots to the strong acid caused instant cell death and converted all DIC and PIC to CO2. DI14C background was degassed in a custom-built desiccator for several days until samples were dry. Deionized water (1 mL) was then added to re-suspend samples prior to addition of 10 mL of scintillation cocktail (Ultima Gold AB, GMI, Ramsey, MN, USA), and the sample was vortexed thoroughly.
Acid-stable (i.e., organic) 14C activity in samples was counted with a Packard Tri-Carb Liquid Scintillation Counter (GMI). Blank samples, consisting of cell-free medium, were treated alongside the other samples. In the few cases where no blanks were available, time zero values were approximated by extrapolating the y-axis intercept from linear fitting of the first three data points of the 14C incorporation curves. Total radioactivity of the NaH14CO3 stock solution was regularly quantified and compared to expected values to estimate loss of radioactivity or changes in counting efficiency. In all spike solutions, measured radioactivity ranged between 80 and 100 % of the theoretical values, and the actual radioactivity levels were used in the calculation of the specific activities. Blank-corrected data were fitted (Eq. 1), using a least-squares-fitting procedure. Applied fit parameters are given in Table 2. Furthermore, a detailed Excel spread sheet for calculating the fit parameters in dependence of the applied conditions (e.g., pH, temperature and DIC concentrations) is provided as Supplementary Material. Please note that in the calculation of initial and final specific activities, we accounted not only for changes in concentrations of 14Ci species but also for changes in concentrations of DI12C, 12CO2, and H12CO3 − upon spike addition. If these changes are neglected, \(\Delta {\text{SA}}_{{{\text{CO}}_{2} }} / {\text{SA}}_{\text{DIC}}\) will be significantly overestimated, leading to an underestimation of \(f_{{{\text{CO}}_{ 2} }}\) (Eq. 1, Table 2, Supplementary material).
We used a numerical sensitivity study to examine how offsets in parameters such as pH, DIC concentrations, radioactivity, temperature, or blank values influence the derived estimates of \(f_{{{\text{CO}}_{ 2} }}\). First, theoretical 14C incorporation curves for "HCO3 − users" \(\left( {f_{{{\text{CO}}_{ 2} }} = 0.25} \right)\) and "CO2 users" \(\left( {f_{{{\text{CO}}_{ 2} }} = 0.80} \right)\) were generated for two assay pH values (7.90 and 8.50) and used as a reference, assuming fixed values of DIC concentrations of 2,300 μmol kg−1, assay temperature of 15 °C, spike solution temperature of 23 °C and spike radioactivity of 370 kBq. In a second step, model fits were obtained using slight offsets in these parameters (e.g., pH 7.95 and 7.85 instead of 7.90) to obtain the effect of parameter variability on \(f_{{{\text{CO}}_{ 2} }}\) estimates. Sensitivity toward over- and underestimation of pH, temperature, DIC concentration, and radioactivity was tested. We further assessed the effects of blank values (±100 dpm) on \(f_{{{\text{CO}}_{ 2} }}\) estimates as a function of different final 14C incorporation rates.
Statistics
All experiments were performed using at least biological triplicates (i.e., three independent, but equally treated cultures). When data were normally distributed (Shapiro-Wilk test) and showed equal variance (Equal-Variance Test), significance in difference between pCO2 treatments was tested by performing student′s t-tests. When samples were not normally distributed or did not show equal variance, a rank sum test was performed instead. Null hypotheses were rejected when p ≤ 0.05, unless otherwise indicated.
Results
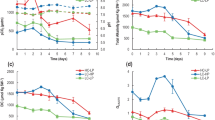
In diploid cells of E. huxleyi, the specific growth rate μ and PIC quotas did not change significantly in response to elevated pCO2 (Table 3). While there was a small decrease in PIC production rates (−11 %), POC quotas and production rates increased strongly under elevated pCO2 (+77 and +55 %, respectively). In conjunction with these changes, the quotas and production rates of TPC also increased (+28 and +23 %, respectively). The PIC:POC ratios of diploid cells decreased from 1.4 to 0.7 under elevated pCO2, while the POC:PON ratios increased from 6.3 to 8.8. Chl a quotas were largely unaffected by the pCO2 treatments, although Chl a:POC ratios decreased significantly from 0.022 to 0.012 pg pg−1 under elevated pCO2, owing to the change in POC quotas. In haploid cells, neither μ, elemental quotas or the respective production rates showed any significant response to elevated pCO2 (Table 3). Similarly, Chl a quotas, Chl a:POC, and POC:PON ratios were all unaffected by the experimental CO2 manipulations in the haploid strain.
Under both pCO2 acclimations, diploid cells were shown to be predominant "CO2 users" under low assay pH (\(f_{{{\text{CO}}_{ 2} }}\) ~ 1.0 at pH 7.9; Fig. 2a). With increasing assay pH, however, we observed a significant increase in relative HCO3 − utilization. HCO3 − uptake was induced at assay pH ≥ 8.3 (equivalent to CO2 concentrations ≤ 9 μmol L−1), reaching considerable contribution at high assay pH (\(f_{{{\text{CO}}_{ 2} }}\) ~ 0.44 at pH 8.7). In contrast to the strong effect of the assay pH, the tested pCO2 acclimations had no effect on the pH-dependent Ci uptake behavior (Fig. 2a). In other words, both low and high pCO2-acclimated cells showed the same short-term response of \(f_{{{\text{CO}}_{ 2} }}\) to assay pH. Like the diploid stage, haploid cells progressively changed from high CO2 usage at low assay pH (\(f_{{{\text{CO}}_{ 2} }}\) ~ 0.96 at pH 7.9) to substantial HCO3 − contributions when assays were conducted in high pH assay buffers (\(f_{{{\text{CO}}_{ 2} }}\) ~ 0.55 at pH 8.5; Fig. 2b). HCO3 − uptake became relevant at pH ≥ 8.1 (equivalent to CO2 concentrations ≤ 14 μmol L−1), particularly in low pCO2-acclimated cells. Except for haploid cells measured at pH 8.1, no significant differences in \(f_{{{\text{CO}}_{ 2} }}\) were observed between the low and high pCO2 acclimations (Fig. 2b).
The sensitivity analysis showed that an offset in the input pH of the buffered assay cell suspension (± 0.05 pH units) led to deviations in \(f_{{{\text{CO}}_{ 2} }}\) of ≤ 0.09 (i.e., 9 percentage points) in "CO2 users" and ≤ 0.02 in "HCO3 − users" (Fig. 3a). An offset in the input temperature of the assay buffer (± 2 °C) led to a deviation in \(f_{{{\text{CO}}_{ 2} }}\) of ≤ 0.09 in "CO2 users" and ≤ 0.03 in "HCO3 − users" (Fig. 3a). An offset in the input pH of the spike (± 0.05 pH units) changed the \(f_{{{\text{CO}}_{ 2} }}\) estimates by ≤ 0.08 in "CO2 users" and ≤ 0.03 in "HCO3 − users" (Fig. 3a). Applying an offset in the input temperature of the spike (± 2 °C) caused a deviation in \(f_{{{\text{CO}}_{ 2} }}\) by ≤ 0.06 in "CO2 users" and had practically no effect on \(f_{{{\text{CO}}_{ 2} }}\) in "HCO3 − users" (≤ 0.01; Fig. 3a). An offset in the input DIC concentration of the buffer (± 100 μmol kg−1) affected \(f_{{{\text{CO}}_{ 2} }}\) by ≤ 0.08 in "CO2 users" and ≤ 0.03 in "HCO3 − users". Regarding the radioactivity of the spike (± 37 kBq), deviations in \(f_{{{\text{CO}}_{ 2} }}\) were ≤ 0.12 in "CO2 users" and ≤ 0.04 in "HCO3 − users." Irrespective of CO2 or HCO3 − usage, offsets in blank estimations (± 100 dpm) led to deviating \(f_{{{\text{CO}}_{ 2} }}\) by ≤ 0.27, but only when equilibrium 14C fixation rates were ≤ 1 dpm s−1 (Fig. 3b). When steady-state 14C incorporation rates were ≥ 2 dpm s−1 (i.e., average rate in diploid cells) and ≥ 4 dpm s−1 (i.e., average rate in haploid cells), the deviations in \(f_{{{\text{CO}}_{ 2} }}\) due to offsets in the blanks were ≤ 0.17 and ≤ 0.11, respectively.
Sensitivity in \(f_{{{\text{CO}}_{ 2} }}\) estimates for "CO2 users" (\(f_{{{\text{CO}}_{ 2} }} = 0.80\)) and "HCO3 − users" (\(f_{{{\text{CO}}_{ 2} }} = 0.25\)) at low pH (7.9, in gray) and high pH (8.5, in white) A toward negative (inverted filled triangle) and positive (filled triangle) offsets in the pH, temperature, and DIC concentration of the assay buffer (pHAssay, T Assay, and [DIC]), as well as toward offsets pH, temperature, and radioactivity of the spike (pHSpike, T Spike, and RA), and B toward negative (inverted filled triangle) and positive (filled triangle) offsets in blank measurements (±100 dpm) in dependence of the final 14C incorporation rates. Sensitivity was assessed based on theoretical curves with constraints of a [DIC]Assay = 2,300 μM, T Assay = 15 °C, T Spike = 23 °C, and RASpike = 37 kBq. Dashed lines indicate \(f_{{{\text{CO}}_{ 2} }}\) values as expected for optimal experimental conditions
Discussion
Acclimation responses
This study corroborates previous findings on the general sensitivity of the diploid life-cycle stage of E. huxleyi toward OA (e.g., Feng et al. 2008; Langer et al. 2009; Riebesell et al. 2000). While growth rate was unaffected, OA reduced PIC production and stimulated POC production (Table 3). Consequently, the PIC:POC ratio was strongly decreased under OA, indicating a redirection of Ci fluxes between these two processes. Transcriptomics have previously attributed this redirection to an inhibition of calcification in response to impaired signal-transduction and ion-transport, as well as to stimulation in the production of glycoconjugates and lipids (Rokitta et al. 2012). In our study, also the TPC production increased significantly under OA (Table 3), indicating that not only Ci is allocated differently, but also the overall Ci uptake increases with the increasing pCO2. Our data further suggest that less energy is required for the Ci acquisition under OA as more POC and TPC could be produced even though the Chl a quota remained unaffected by the pCO2 treatment (Table 3). Improved energy-use efficiencies under OA have previously been proposed for the diploid life-cycle stage of E. huxleyi (Rokitta and Rost 2012).
In strong contrast to the diploid strain, the haploid life-cycle stage of E. huxleyi was insensitive toward OA with respect to growth rate and elemental composition (Table 3). The ability of the haploid cells to maintain homeostasis under OA has also been observed by Rokitta and Rost (2012). Even though the haploid cells appeared non-responsive toward OA on the phenomenological level (i.e., growth, elemental composition), transcriptomics have revealed significant changes at the subcellular level, such as an upregulation of catabolic pathways under OA (Rokitta et al. 2012). Based on the comparison of the life-cycle stages, Rokitta and co-workers concluded that the OA sensitivity in diploid cells originates from calcification, differences in Ci acquisition or both.
A number of studies have shown that E. huxleyi has moderately high Ci affinities and uses HCO3 − as the primary Ci source (e.g., Herfort et al. 2002; Rokitta and Rost 2012; Rost et al. 2006b; Stojkovic et al. 2013), irrespective of the degree of calcification (Trimborn et al. 2007; Rokitta and Rost 2012). These characteristics would suggest E. huxleyi to be rather insensitive toward OA and the associated rise in CO2 concentration, contrary to most results obtained for the diplont. As discussed below, this apparent discrepancy could originate from differences in conditions applied during short-term physiological measurements and those conditions cells experience in the long-term acclimation.
Modes of Ci acquisition
Our results demonstrate that the Ci source of both life-cycle stages of E. huxleyi is significantly influenced by the pH of the assay medium and the resulting carbonate chemistry (Fig. 2). With increasing pH in assay buffers, cells progressively changed from predominant CO2 usage at lower pH values (≤ 8.1) to significant HCO3 − contribution at higher pH (≥ 8.3). Surprisingly, this change occurred irrespectively of the pCO2 conditions in the acclimation. To our knowledge, such a strong short-term pH-dependence in Ci acquisition has not been previously reported, which is most likely due to the fact that assays are typically performed under standardized pH values. Measuring physiological responses under one reference condition have the advantage that consequences of different acclimations can readily be compared in terms of altered capacities of certain processes, e.g., enzyme activities or transport rates. However, determination of the Ci source at one standard pH appears to impose a methodological bias, and our results, therefore, bear direct relevance to the interpretation of previous laboratory observations.
In view of the short-term pH effect on Ci acquisition, the contribution of HCO3 − as a photosynthetic Ci source in E. huxleyi may have possibly been overestimated in previous studies. This overestimation is likely to be the most significant in those studies when 14C disequilibrium assays were conducted at pH 8.5 (e.g., Rokitta and Rost 2012; Rost et al. 2007). By looking at the Ci source determined at an assay pH mimicking the acclimation condition, we can now re-evaluate and in fact explain the responses of E. huxleyi toward elevated pCO2. When assessing \(f_{{{\text{CO}}_{ 2} }}\) using assay buffers of pH 7.9 and 8.1 (equivalent to the acclimation pH of high and low pCO2 treatments), we observed predominant CO2 uptake under both conditions (Fig. 2). Being "CO2 user", cells were thus able to directly benefit from changes in the CO2 concentrations in our acclimations (~15 μmol kg−1 at 380 μatm and ~38 μmol kg−1 at 950 μatm). For a "HCO3 − user", however, it would be difficult to argue for a beneficial OA-effect as HCO3 − concentrations do not differ much between treatments (~1,930 μmol kg−1 at 380 μatm and ~2,130 μmol kg−1 at 950 μatm). Our results thus suggest that biomass production in diploid cells not only profits from the declined calcification at high pCO2, as suggested by Rokitta and Rost (2012) but also from the higher CO2 supply under OA. As CO2 usage is considered to be less costly than HCO3 − uptake (Raven 1990), this could also explain the higher energy-use efficiency observed for E. huxleyi (Rokitta and Rost 2012).
Although the haploid life-cycle stage of E. huxleyi exhibited a pH-dependent Ci uptake behavior that was similar to the diploid (Fig. 2), the haploid cells did not show any CO2-dependent stimulation in biomass production (Table 3). This could partly be related to the fact that the biomass production cannot profit from a down-scaling of calcification, simply because this process is absent in the haploid life-cycle stage. The lack of significantly stimulated biomass buildup under OA could also be attributed to the concomitant upregulation of catabolic pathways, such as higher lipid consumption, which is a specific feature of the haploid cells (Rokitta et al. 2012). After all, the similar Ci uptake behavior of both life-cycle stages confirms that photosynthetic HCO3 − usage is not tied to calcification (Herfort et al. 2004; Trimborn et al. 2007; Bach et al. 2013) and that the preference for CO2 or HCO3 − is predominantly controlled by carbonate chemistry.
Our findings clearly demonstrate that the acclimation history, in both life-cycle stages, has little or no effect on the Ci usage of the cells (Fig. 2). In other words, the instantaneous effect of the assay conditions dominates over acclimation effects. We cannot preclude, however, that cells acclimated to higher pH values, where CO2 supply becomes limiting, may increase their capacity for HCO3 − uptake and acclimations effects would then be evident. Notwithstanding the potential for some acclimation effects, the extent to which short-term pH and/or CO2 levels in the assay medium directly control cellular Ci usage is striking. This implies that even though E. huxleyi did not use significant amounts of HCO3 − for photosynthesis, it must constitutively express a HCO3 − transporter in all acclimations. Without the presence of a functional HCO3 − transport system we could otherwise not explain the capacity for significant HCO3 − uptake under short-term exposure to high pH (even in high pCO2-acclimated cells).
In the diploid life-cycle stage, HCO3 − transporter may be constitutively expressed to fuel calcification, as HCO3 − was identified as the main Ci source for this process (Paasche 1964; Rost et al. 2002; Sikes et al. 1980). If CO2 supply for photosynthesis becomes limiting, HCO3 − transport could then also fuel photosynthesis. In the haploid cells, which do not calcify, we nonetheless observed the same capacity for HCO3 − uptake, which suggests that HCO3 − uptake capacity represents a fundamental component of the CCM of both life-cycle stages of E. huxleyi. Whether levels of protons or CO2 concentrations are the main trigger for the shift between CO2 and HCO3 − uptake remains unclear, even though there is strong evidence that CO2 supply is the main driver for the responses in photosynthesis (Bach et al. 2011).
Sensitivity analyses
In our sensitivity study, the applied offsets in pH (± 0.05 pH units), temperature (± 2 °C), DIC of the assay buffer (± 100 μM), and spike radioactivity (± 37 kBq) were larger than typical measurement errors to represent "worst-case scenarios". None of these offsets caused \(f_{{{\text{CO}}_{ 2} }}\) estimates to deviate by more 0.12 in any of the pH treatments (Fig. 3a). When adequate efforts are taken to control these parameters (e.g., using reference buffers, thermostats), methodological uncertainties are thus negligible. DIC concentrations and radioactivity, however, are often not measured and in view of the potential drift over time, offsets can easily exceed typical measurement errors and lead to severe deviations in \(f_{{{\text{CO}}_{ 2} }}\). For instance, 14CO2 out-gassing causes the spike solution to progressively lose radioactivity. This loss of 14C can easily be > 20 % over the course of weeks or months, despite the high pH values of the stock solution and small headspace in the storage vial (Gattuso et al. 2010).
The average final 14C fixation rates, which depend on the biomass and radioactivity used, were 2.1 ± 0.8 dpm s−1 in the runs with diploid and 6.6 ± 2.2 dpm s−1 in those with haploid cells (Fig. 3b). In these ranges, offsets in blank values (± 100 dpm) can lead to biases in the estimated \(f_{{{\text{CO}}_{ 2} }}\) by up to 0.20 (Fig. 3b). This strong sensitivity highlights the need to thoroughly determine blank values, but also to work with sufficiently high biomass and/or radioactivity to maximize 14C incorporation rates. When working with dense cell suspensions, however, self-shading or significant draw-down of DIC during the assay might bias results. Higher label addition generally increases the resolution of the assay and lowers the consequences of offsets in the blank value. It should be noted, however, that high concentrations of 14C in spike solutions can affect not only the isotopic but also the chemical conditions in the cuvette (e.g., pH and DIC).
Overall, our sensitivity study revealed that the 14C disequilibrium method is a straightforward and robust assay, which is very useful for resolving the Ci source of phytoplankton over a range of different pH values. It is important to realize, however, the pH of assay buffers has the potential to significantly affect the Ci uptake behavior of cells.
Conclusions
Our data clearly demonstrate that both life-cycle stages of E. huxleyi predominantly use CO2 as Ci source for photosynthesis under typical present-day and future CO2 levels, but constitutively express HCO3 − transporters allowing them to directly use HCO3 − when CO2 becomes limiting. Under bloom conditions, where pH values can easily increase to 8.5 or higher, cells might, therefore, be able to maintain efficient Ci acquisition. Future research needs to investigate whether and how the assay pH governs the mode of Ci acquisition also in other coccolithophores species or phytoplankton taxa and how this may alter the energy budget of cells. Results from previous studies may need re-consideration in the light of our data showing strong short-term pH effects on Ci uptake of phytoplankton.
References
Anning T, Nimer N, Merrett M, Brownlee C (1996) Costs and benefits of calcification in coccolithophorids. J Mar Syst 9:45–56
Bach LT, Riebesell U, Schulz KG (2011) Distinguishing between the effects of ocean acidification and ocean carbonation in the coccolithophore Emiliania huxleyi. Limnol Oceanogr 56:2040–2050
Bach LT, Mackinder LCM, Schulz KG, Wheeler G, Schroeder DC, Brownlee C, Riebesell U (2013) Dissecting the impact of CO2 and pH on the mechanisms of photosynthesis and calcification in the coccolithophore Emiliania huxleyi. New Phytol 199:121–134
Badger MR (2003) The roles of carbonic anhydrases in photosynthetic CO2 concentrating mechanisms. Photosynth Res 77:83–94
Broecker WS, Peng T-H (1982) Tracers in the Sea. EldigioPress, New York
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH—the coming centuries may see more oceanic acidification than the past 300 million years. Nature 425:365
Cassar N, Laws EA, Bidigare RR, Popp BN (2004) Bicarbonate uptake by Southern Ocean phytoplankton. Glob Biogeochem Cy 18:GB2003. doi:10.1029/2003GB002116
Dickson AG (1981) An exact definition of total alkalinity and a procedure for the estimation of alkalinity and total inorganic carbon from titration data. Deep Sea Res 28A:609–623
Dickson AG (1990) Standard potential of the reaction: AgCl(s) + ½ H2(g) = Ag(s) + HCl(aq), and the standard acidity constant of the ion HSO4 − in synthetic seawater from 273.15 to 318.15 K. J Chem Thermodyn 22:113–127
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res 34:1733–1743
Elzenga JTM, Prins HBA, Stefels J (2000) The role of extracellular carbonic anhydrase activity in inorganic carbon utilization of Phaeocystis globosa (Prymnesiophyceae): a comparison with other marine algae using the isotopic disequilibrium technique. Limnol Oceanogr 45:372–380
Espie GS, Colman B (1986) Inorganic carbon uptake during photosynthesis—a theoretical analysis using the isotopic disequilibrium technique. Plant Physiol 80:863–869
Falkowski PG, Barber RT, Smetacek V (1998) Biogeochemical controls and feedbacks on ocean primary production. Science 281:200–206
Feng Y, Warner ME, Zhang Y, Sun J, Fu FX, Rose JM, Hutchins DA (2008) Interactive effects of increased pCO2, temperature and irradiance on the marine coccolithophore Emiliania huxleyi (Prymnesiophyceae). Eur J Phycol 43:87–98
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere—integrating terrestrial and oceanic components. Science 281:237–240
Frada MJ, Bidle KD, Probert I, de Vargas C (2012) In situ survey of life cycle phases of the coccolithophore Emiliania huxleyi (Haptophyta). Environ Microbiol 14(6):1558–1569
Gattuso J-P, Gao K, Lee K, Rost B, Schulz K (2010). Approaches and tools to manipulate the carbonate chemistry, In: Riebesell U, Fabry VJ, Hansson L, Gattuso J-P (eds) Guide for best practices in ocean acidification research and data reporting. European Commission, pp 41–52
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. Can J Microbiol 8:229–239
Herfort L, Thake B, Roberts J (2002) Acquisition and use of bicarbonate by Emiliania huxleyi. New Phytol 156:427–436
Herfort L, Loste E, Meldrum F, Thake B (2004) Structural and physiological effects of calcium and magnesium in Emiliania huxleyi (Lohmann) Hay and Mohler. J Struct Biol 148:307–314
Holm-Hansen O, Riemann B (1978) Chlorophyll a determination: improvements in methodology. Oikos 30:438–447
Hoppe CJM, Langer G, Rost B (2011) Emiliania huxleyi shows identical responses to elevated pCO2 in TA and DIC manipulations. J Exp Mar Biol Ecol 406:54–62
Hoppe CJM, Langer G, Rokitta SD, Wolf-Gladrow DA, Rost B (2012) Implications of observed inconsistencies in carbonate chemistry measurements for ocean acidification studies. Biogeosciences 9:2401–2405
Johnson KS (1982) Carbon dioxide hydration and dehydration kinetics in seawater. Limnol Oceanogr 27:849–855
Langer G, Nehrke G, Probert I, Ly J, Ziveri P (2009) Strain-specific responses of Emiliania huxleyi to changing seawater carbonate chemistry. Biogeosciences 6:2637–2646
Mackinder L, Wheeler G, Schroeder D, Riebesell U, Brownlee C (2010) Molecular mechanisms underlying calcification in coccolithophores. Geomicrobiol J 27:585–595
Martin CL, Tortell PD (2006) Bicarbonate transport and extracellular carbonate anhydrase in Bering Sea phytoplankton assemblages: results from isotopic disequilibrium experiments. Limnol Oceanogr 51:2111–2121
Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18:897–907
Millero FJ, Roy RN (1997) A chemical equilibrium model for the carbonate system in natural waters. Croat Chem Acta 70:1–38
Paasche E (1964) A tracer study of the inorganic carbon uptake during coccolith formation and photosynthesis in the coccolithophorid Coccolithus huxleyi. Physiol Plant 18:138–145
Paasche E (2002) A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions. Phycologia 40:503–529
Pierrot D, Lewis E, Wallace D (2006) MS Excel program developed for CO2 system calculations. ORNL/CDIAC-105 Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge
Raven JA (1990) Sensing pH? Plant Cell Environ 13:721–729
Raven JA (2006) Sensing inorganic carbon: CO2 and HCO3 −. Biochem J 396:e5–e7. doi:10.1042/BJ20060574
Raven JA, Crawfurd K (2012) Environmental controls on coccolithophore calcification. Mar Ecol Prog Ser 470:137–166
Read BA, Kegel J, Klute MJ, Kuo A, Lefebvre SC, Maumus F, Mayer C, Miller J, Monier A, Salamov A et al (2013) Pan genome of the phytoplankton Emiliania underpins its global distribution. Nature 499:209–213
Reinfelder JR (2011) Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annu Rev Mar Sci 3:291–315
Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe E, Morel FMM (2000) Reduced calcification in marine plankton in response to increased atmospheric CO2. Nature 407:364–367
Rokitta SD, Rost B (2012) Effects of CO2 and their modulation by light in the life-cycle stages of the coccolithophore Emiliania huxleyi. Limnol Oceanogr 57(2):607–618
Rokitta SD, De Nooijer LJ, Trimborn S, De Vargas C, Rost B, John U (2011) Transcriptome analyses reveal differential gene expression patterns between lifecycle stages of Emiliania huxleyi (Haptophyta) and reflect specialization to different ecological niches. J Phycol 47:829–838
Rokitta SD, John U, Rost B (2012) Ocean acidification affects redox-balance and ion-homeostasis in the life-cycle stages of Emiliania huxleyi. PLoS One 7(12):e52212. doi:10.1371/journal.pone.0052212
Rost B, Zondervan I, Riebesell U (2002) Light-dependent carbon isotope fractionation in the coccolithophorid Emiliania huxleyi. Limnol Oceanogr 47:120–128
Rost B, Riebesell U, Burkhardt S, Sültemeyer D (2003) Carbon acquisition of bloom-forming marine phytoplankton. Limnol Oceanogr 48:55–67
Rost B, Richter K-U, Riebesell U, Hansen PJ (2006a) Inorganic carbon acquisition in red tide dinoflagellates. Plant Cell Environ 29:810–822
Rost B, Riebesell U, Sültemeyer D (2006b) Carbon acquisition of marine phytoplankton: effect of photoperiod length. Limnol Oceanogr 51:12–20
Rost B, Kranz SA, Richter KU, Tortell PD (2007) Isotope disequilibrium and mass spectrometric studies of inorganic carbon acquisition by phytoplankton. Limnol Oceanogr Methods 5:328–337
Sikes CS, Roer RD, Wilbur KM (1980) Photosynthesis and coccolith formation: inorganic carbon sources and net inorganic reaction of deposition. Limnol Oceanogr 25:248–261
Stojkovic S, Beardall J, Matear R (2013) CO2-concentrating mechanisms in three southern hemisphere strains of Emiliania huxleyi. J Phycol 49:670–679
Stoll MHC, Bakker K, Nobbe GH, Haese AR (2001) Continuous-flow analysis of dissolved inorganic carbon content in seawater. Anal Chem 73:4111–4116
Suffrian K, Schulz KG, Gutowska MA, Riebesell U, Bleich M (2011) Cellular pH measurements in Emiliania huxleyi reveal pronounced membrane proton permeability. New Phytol 190:595–608
Taylor AR, Chrachi A, Wheeler G, Goddard H, Brownlee C (2011) A voltage-gated H+ channel underlying pH homeostasis in calcifying coccolithophores. PLoS Biol 9(6):14–16
Tortell PD, Morel FMM (2002) Sources of inorganic carbon for phytoplankton in the eastern Subtropical and Equatorial Pacific Ocean. Limnol Oceanogr 47:1012–1022
Tortell PD, Payne CD, Li Y, Trimborn S, Rost B, Smith WO, Riesselman C, Dunbar R, Sedwick P, DiTullio G (2008) The CO2 response of Southern Ocean phytoplankton. Geophys Res Lett 35:L04605
Trimborn S, Langer G, Rost B (2007) Effect of varying calcium concentrations and light intensities on calcification and photosynthesis in Emiliania huxleyi. Limnol Oceanogr 52:2285–2293
Westbroek P, Brown CW, Van Bleijswijk J, Brownlee C, Brummer GJ, Conte M, Egge J, Fernandez E, Jordan R, Knappertsbusch M, Stefels J, Veldhuis M, Van Der Wal P, Young J (1993) A model system approach to biological climate forcing—the example of Emiliania huxleyi. Glob Planet Change 8:27–46
Wolf-Gladrow DA, Riebesell U, Burkhardt S, Bijma J (1999) Direct effects of CO2 concentration on growth and isotopic composition of marine plankton. Tellus 51:461–476
Zeebe RE, Wolf-Gladrow DA (2007) CO2 in seawater: equilibrium, kinetics, isotopes. Elsevier Science B.V, Amsterdam
Acknowledgments
We thank Silke Thoms and Lena Holtz for the discussion of our data and their constructive feedback on this manuscript. This work was supported by the European Community’s Seventh Framework Programme/ERC grant agreement #205150, and by an Alexander Von Humboldt fellowship to PDT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: James Moroney.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kottmeier, D.M., Rokitta, S.D., Tortell, P.D. et al. Strong shift from HCO3 − to CO2 uptake in Emiliania huxleyi with acidification: new approach unravels acclimation versus short-term pH effects. Photosynth Res 121, 265–275 (2014). https://doi.org/10.1007/s11120-014-9984-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-9984-9