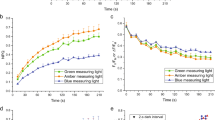
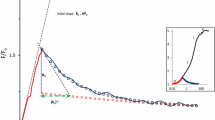
Abstract
The operation of photosynthetic energy-dissipating processes is commonly characterized by measuring the light response of the nonphotochemical quenching (NPQ) of chlorophyll fluorescence, or NPQ versus E curves. This study proposes a mathematical model for the quantitative description of the generic NPQ versus E curve. The model is an adaptation of the Hill equation and is based on the close dependence of NPQ on the xanthophyll cycle (XC). The model was tested on NPQ versus E curves measured in the plant Arabidopsis thaliana and the diatom Nitzschia palea, representing the two main types of XC, the violaxanthin–antheraxanthin–zeaxanthin (VAZ) type and the diadinoxanthin–diatoxanthin (DD–DT) type, respectively. The model was also fitted to a large number of published light curves, covering the widest possible range of XC types, taxa, growth conditions, and experimental protocol of curve generation. The model provided a very good fit to experimental and published data, coping with the large variability in curve characteristics. The model was further used to quantitatively compare the light responses of NPQ and of PSII electron transport rate, ETR, through the use of indices combining parameters of the models describing the two types of light–response curves. Their application to experimental and published data showed a systematic large delay of the buildup of NPQ relatively to the saturation of photochemistry. It was found that when ETR reaches saturation, NPQ is on average still below one fifth of its maximum attainable level, which is only reached at irradiances about three times higher. It was also found that organisms having the DD–DT type of XC appeared to be able to start operating the XC at lower irradiances than those of the VAZ type.






Similar content being viewed by others
Abbreviations
- α:
-
Initial slope of the ETR versus E curve
- Ax:
-
Antheraxanthin
- DD:
-
Diadinoxanthin
- DT:
-
Diatoxanthin
- E :
-
PAR irradiance (μmol m−2 s−1)
- E 50 :
-
Irradiance level corresponding to 50% of NPQm in a NPQ versus E curve
- E k :
-
Light-saturation parameter of the ETR versus E curve
- ETR:
-
PSII relative electron transport rate
- ETRm :
-
Maximum ETR in a ETR versus E curve
- Fo, Fm:
-
Minimum and maximum fluorescence of a dark-adapted sample
- Fs, \( F_{\text{m}}^{'} \):
-
Steady state and maximum fluorescence of a light-adapted sample
- NPQ:
-
Nonphotochemical quenching
- NPQm :
-
Maximum NPQ value reached in a NPQ versus E curve
- \( {\text{NPQ}}_{{E_{\text{k}} }} \) :
-
Fraction of NPQm reached when E = E k
- n :
-
Sigmoidicity coefficient of the NPQ versus E curve
- PSII:
-
Photosystem II
- RLC:
-
Rapid light–response curve
- VAZ:
-
Vx–Ax–Zx XC
- Vx:
-
Violaxanthin
- XC:
-
Xanthophyll cycle
- Zx:
-
Zeaxanthin
References
Bailleul B, Rogato A, Martino A, Coesel S, Cardol P, Bowler C, Falciatore A, Finazzi G (2010) An atypical member of the light-harvesting complex stress-related protein family modulates diatom responses to light. Proc Natl Acad Sci USA 107:18214–18219
Baker NR, Oxborough K (2004) Chlorophyll fluorescence as probe of plant photosynthetic productivity. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 65–82
Behrenfeld MJ, Prasil O, Babin M, Bruyant F (2004) In search of a physiological basis for covariations in light-limited and light-saturated photosynthesis. J Phycol 40:4–25
Bilger W, Bjorkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Burritt DJ, Mackenzie S (2003) Antioxidant metabolism during acclimation of Begonia × erytrophylla to high light levels. Ann Bot 91:783–794
Campbell D, Hurry V, Clarke AK, Gustafsson P, Gunnar Ö (1998) Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Molec Biol Rev 62:667–683
Cosgrove J, Borowitzka M (2006) Applying pulse amplitude modulation (PAM) fluorometry to microalgae suspensions: stirring potentially impacts fluorescence. Photosynth Res 88:343–350
Cruz S, Serôdio J (2008) Relationship of rapid light curves of variable fluorescence to photoacclimation and non-photochemical quenching in a benthic diatom. Aquat Bot 88:256–264
D’Haese D, Vandermeiren K, Caubergs RJ, Guisez Y, De Temmerman L, Horemans N (2004) Non-photochemical quenching kinetics during the dark to light transition in relation to the formation of antheraxanthin and zeaxanthin. J Theor Biol 227:175–186
Demmig-Adams B, Adams WW (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172:11–21
Dimier C, Corato F, Saviello G, Brunet C (2007a) Photophysiological properties of the marine picoeukaryote Picochlorum Rcc 237 (Trebouxiophyceae, Chlorophyta). J Phycol 43:275–283
Dimier C, Corato F, Tramontano F, Brunet C (2007b) Photoprotection and xanthophyll-cycle activity in three marine diatoms. J Phycol 43:937–947
Eberhard S, Finazzi G, Wollman F (2008) The dynamics of photosynthesis. Ann Rev Gen 42:463–515
Eilers PH, Peeters JC (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Model 42:199–215
Elrad D, Niyogi KK, Grossman AR (2002) A major light-harvesting polypeptide of photosystem II functions in thermal dissipation. Plant Cell 14:1801–1816
Geel C, Verluis W, Snel JF (1997) Estimation of oxygen evolution by marine phytoplankton from measurement of the efficiency of photosystem II electron flow. Photosynth Res 51:61–70
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Goss R, Jakob T (2010) Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth Res 106:103–122
Goss R, Pinto EA, Wilhelm C, Richter M (2006) The importance of a highly active and ΔpH-regulated diatoxanthin epoxidase for the regulation of the PS II antenna function in diadinoxanthin cycle containing algae. J Plant Physiol 163:1008–1021
Guarini J, Moritz C (2009) Modelling the dynamics of the electron transport rate measured by PAM fluorimetry during rapid light curve experiments. Photosynthetica 47:206–214
Guillard RRL, Ryther JH (1962) Studies of marine phytoplanktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervaceae (Cleve) Gran. Can J Microbiol 8:229–239
Havaux M, Kloppstech K (2001) The protective functions of carotenoid and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta 213:953–966
Henley WJ (1993) Measurement and interpretation of photosynthetic light–response curves in algae in the context of photoinhibition and diel changes. J Phycol 29:729–739
Herlory O, Richard P, Blanchard GF (2007) Methodology of light response curves: application of chlorophyll fluorescence to microphytobenthic biofilms. Mar Biol 153:91–101
Horton P, Ruban AV, Wentworth M (2000) Allosteric regulation of the light-harvesting system of photosystem II. Philos Trans Royal Soc London Series B 355:1361–1370
Horton P, Johnson MP, Perez-Bueno ML, Kiss AZ, Ruban AV (2008) Photosynthetic acclimation: does the dynamic structure and macro-organisation of photosystem II in higher plant grana membranes regulate light harvesting states? FEBS J 275:1069–1079
Jakob T, Goss R, Wilhelm C (1999) Activation of diadinoxanthin de-epoxidase due to a chlororespiratory proton gradient in the dark in the diatom Phaeodactylum tricornutum. Plant Biol 1:76–82
Johnson ML (2008) Nonlinear least-squares fitting methods. In: Correia J, Detrich H (eds) Methods in cell biology, vol 84. Elsevier Academic Press, San Diego, pp 781–805
Jung H-S, Niyogi KK (2009) Quantitative genetic analysis of thermal dissipation in Arabidopsis. Plant Physiol 150:977–986
Kromkamp JC, Dijkman NA, Peene J, Simis SGH, Gons HJ (2008) Estimating phytoplankton primary production in Lake IJsselmeer (The Netherlands) using variable fluorescence (PAM-FRRF) and C-uptake techniques. Eur J Phycol 43:327–344
Kropuenske LR, Mills MM, Dijken GL, Bailey S, Robinson DH, Welschmeyer NA, Arrigo KR (2009) Photophysiology in two major Southern Ocean phytoplankton taxa: photoprotection in Phaeocystis antarctica and Fragilariopsis cylindrus. Limnol Oceanogr 54:1176–1196
Krupenina NA, Bulychev AA (2007) Action potential in a plant cell lowers the light requirement for non-photochemical energy-dependent quenching of chlorophyll fluorescence. Biochim Biophys Acta 1767:781–788
Lavaud J (2007) Fast regulation of photosynthesis in diatoms: mechanisms, evolution and ecophysiology. Funct Plant Sci Biotechnol 1:267–287
Lavaud J, Kroth PG (2006) In diatoms, the transthylakoid proton gradient regulates the photoprotective non-photochemical fluorescence quenching beyond its control on the xanthophyll cycle. Plant Cell Physiol 47:1010–1016
Lavaud J, Rousseau B, Gorkom HJ, Etienne A (2002) Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom. Plant Physiol 129:1398–1406
Lavaud J, Rousseau B, Etienne AL (2004) General features of photoprotection by energy dissipation in planktonic diatoms (Bacillariophyceae). J Phycol 40:130–137
Lavaud J, Strzepek RF, Kroth PG (2007) Photoprotection capacity differs among diatoms: possible consequences on the spatial distribution of diatoms related to fluctuations in the underwater light climate. Limnol Oceanogr 52:1188–1194
Li X-P, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403:391–395
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Lokstein H, Tian L, Polle JE, DellaPenna D (2002) Xanthophyll biosynthetic mutants of Arabidopsis thaliana: altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in photosystem II antenna size and stability. Biochim Biophys Acta 1553:309–319
Marschall M, Proctor MCF (2004) Are bryophytes shade plants? Photosynthetic light responses and proportions of chlorophyll a, chlorophyll b and total carotenoids. Ann Bot 94:593–603
Mouget J-L, Tremblin G (2002) Suitability of the fluorescence monitoring system (FMS, Hansatech) for measurement of photosynthetic characteristics in algae. Aquat Bot 74:219–231
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Müller-Moulé P, Conklin PL, Niyogi KK (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128:970–977
Müller-Moulé P, Golan T, Niyogi KK (2004) Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol 134:1163–1172
Mullineaux CW, Emlyn-Jones D (2005) State transitions: an example of acclimation to low-light stress. J Exp Bot 56:389–393
Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110:361–371
Munshi MK, Kobayashi Y, Shikanai T (2006) Chlororespiratory reduction 6 is a novel factor required for accumulation of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Physiol 141:737–744
Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10:1121–1134
Olaizola M, Yamamoto HY (1994) Short-term response of the diadinoxanthin cycle and fluorescence yield to high irradiance in Chaetoceros muelleri (Bacillariophyceae). J Phycol 30:606–612
Ort DR (2001) When there is too much light. Plant Physiol 125:29–32
Osmond B, Badger M, Maxwell K, Björkman O, Leegood R (1997) Too many photons: photorespiration, photoinhibition and photooxidation. Trends Plant Sci 2:119–121
Peers G, Truong TB, Ostendor E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462:518–522
Pérez-Bueno ML, Johnson MP, Zia A, Ruban AV, Horton P (2008) The Lhcb protein and xanthophyll composition of the light harvesting antenna controls the ΔpH-dependency of non-photochemical quenching in Arabidopsis thaliana. FEBS Lett 582:1477–1482
Perkins RG, Mouget J-L, Lefebvre S, Lavaud J (2006) Light response curve methodology and possible implications in the application of chlorophyll fluorescence to benthic diatoms. Mar Biol 149:703–712
Perkins RG, Kromkamp J, Serôdio J, Lavaud J, Jesus B, Mouget J-L, Forster R, Lefebvre S (2010) The application of variable chlorophyll fluorescence to microphytobenthic biofilms. In: Suggett D, Prasil O, Borowitza MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Developments in applied phycology, 4th edn. Springer, Dordrecht, pp 273–275
Pfannschmidt T (2005) Acclimation to varying light qualities: toward the functional relationship of state transitions and adjustment of photosystem stoichiometry. J Phycol 41:723–725
Pfündel EE, Dilley RA (1993) The pH dependence of violaxanthin deepoxidation in isolated pea chloroplasts. Plant Physiol 101:65–71
Press WH, Teukolsky S, Vetterling W, Flannery B (1996) Numerical recipes in Fortran 90. The art of parallel scientific computing, 2nd edn. Cambridge University Press, Cambridge
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Ralph PJ, Gademann R, Larkum AW (2001) Zooxanthellae expelled from bleached corals at 33°C are photosynthetically competent. Mar Ecol Prog Ser 220:163–168
Ritchie RJ (2008) Fitting light saturation curves measured using modulated fluorometry. Photosynth Res 96:201–215
Rodríguez-Calcerrada J, Pardos JA, Gil L, Aranda I (2007) Acclimation to light in seedlings of Quercus petraea (Mattuschka) Liebl. and Quercus pyrenaica Willd. planted along a forest-edge gradient. Trees 21:45–54
Ruban AV, Johnson MP (2009) Dynamics of higher plant photosystem cross-section associated with state transitions. Photosynth Res 99:173–183
Ruban AV, Wentworth M, Horton P (2001) Kinetic analysis of nonphotochemical quenching of chlorophyll fluorescence. 1. Isolated chloroplasts. Biochemistry 40:9896–9901
Serôdio J, Vieira S, Cruz S, Coelho H (2006) Rapid light–response curves of chlorophyll fluorescence in microalgae: relationship to steady-state light curves and non-photochemical quenching in benthic diatom-dominated assemblages. Photosynth Res 90:29–43
Takizawa K, Cruz JA, Kanazawa A, Kramer DM (2007) The thylakoid proton motive force in vivo. Quantitative, noninvasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim Biophys Acta 1767:1233–1244
Tellinghuisen J (2008) Stupid statistics!. In: Correia J, Detrich H (eds) Methods in cell biology, vol 84. Elsevier Academic Press, San Diego, pp 739–780
Tikkanen M, Pippo M, Suorsa M, Sirpio S, Mulo P, Vainonen J, Vener A, Allahverdiyeva Y, Aro EM (2006) State transitions revisited: a buffering system for dynamic low light acclimation of Arabidopsis. Plant Molec Biol 62:779–793
Voet D, Voet JG (1990) Biochemistry. John Wiley and Sons, New York
Acknowledgments
We thank Glória Pinto, Eleazar Rodriguez, and Armando Costa for helping with the seeding of the Arabidopsis plants. This study was supported by FCT—Fundação para a Ciência e a Tecnologia, grant SFRH/BSAB/962/2009 to J. Serôdio, by the French consortium CPER-Littoral to J. Lavaud, and by the CNRS—Centre National de la Recherche Scientifique (programme ‘chercheurs invités’) to both. We thank two anonymous reviewers for critical comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Serôdio, J., Lavaud, J. A model for describing the light response of the nonphotochemical quenching of chlorophyll fluorescence. Photosynth Res 108, 61–76 (2011). https://doi.org/10.1007/s11120-011-9654-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-011-9654-0