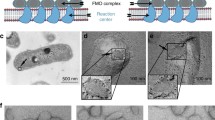
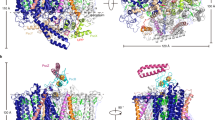
Abstract
In contrast to photosynthetic reaction centers, which share the same structural architecture, more variety is found in the light-harvesting antenna systems of phototrophic organisms. The largest antenna system described, so far, is the chlorosome found in anoxygenic green bacteria, as well as in a recently discovered aerobic phototroph. Chlorosomes are the only antenna system, in which the major light-harvesting pigments are organized in self-assembled supramolecular aggregates rather than on protein scaffolds. This unique feature is believed to explain why some green bacteria are able to carry out photosynthesis at very low light intensities. Encasing the chlorosome pigments is a protein-lipid monolayer including an additional antenna complex: the baseplate, a two-dimensional paracrystalline structure containing the chlorosome protein CsmA and bacteriochlorophyll a (BChl a). In this article, we review current knowledge of the baseplate antenna complex, which physically and functionally connects the chlorosome pigments to the reaction centers via the Fenna–Matthews–Olson protein, with special emphasis on the well-studied green sulfur bacterium Chlorobaculum tepidum (previously Chlorobium tepidum). A possible role for the baseplate in the biogenesis of chlorosomes is discussed. In the final part, we present a structural model of the baseplate through combination of a recent NMR structure of CsmA and simulation of circular dichroism and optical spectra for the CsmA–BChl a complex.





Similar content being viewed by others
References
Adolphs J, Renger T (2006) How proteins trigger excitation energy transfer in the FMO complex of green sulfur bacteria. Biophys J 91:2778–2797. doi:10.1529/biophysj.105.079483
Arellano JB, Psencik J, Borrego CM et al (2000) Effect of carotenoid biosynthesis inhibition on the chlorosome organization in Chlorobium phaeobacteroides strain CL1401. Photochem Photobiol 71:715–723. doi:10.1562/0031-8655(2000)0710715EOCBIO2.0.CO2
Armstrong D, Zidovetzki R (2001) Wheel.Pl. http://rzlab.Ucr.Edu/scripts/wheel/wheel.Cgi
Beatty JT, Overmann J, Lince MT et al (2005) An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. PNAS 102:9306–9310. doi:10.1073/pnas.0503674102
Ben-Shem A, Frolow F, Nelson N (2004) Evolution of photosystem I—from symmetry through pseudo-symmetry to asymmetry. FEBS Lett 564:274–280. doi:10.1016/S0014-5793(04)00360-6
Betti JA, Blankenship RE, Natarajan LV et al (1982) Antenna organization and evidence for the function of a new antenna pigment species in the green photosynthetic bacterium Chloroflexus aurantiacus. Biochim Biophys Acta 680:194–201. doi:10.1016/0005-2728(82)90011-1
Blankenship RE, Matsuura K (2003) Antenna complexes from green photosynthetic bacteria. In: Green BR, Parson WW (eds) Light-harvesting antennas in photosynthesis. Kluwer, Dordrecht, The Netherlands
Bryant DA, Vassilieva EV, Frigaard NU et al (2002) Selective protein extraction from Chlorobium tepidum chlorosomes using detergents. Evidence that CsmA forms multimers and binds bacteriochlorophyll a. Biochemistry 41:14403–14411. doi:10.1021/bi026599s
Bryant DA, Costas AM, Maresca JA et al (2007) “Candidatus Chloracidobacterium thermophilum”: an aerobic phototrophic acidobacterium. Science 317:523–526. doi:10.1126/science.1143236
Camara-Artigas A, Blankenship RE, Allen JP (2003) The structure of the FMO protein from Chlorobium tepidum at 2.2 angstrom resolution. Photosynth Res 75:49–55. doi: 10.1023/A:1022406703110
Chung S, Bryant DA (1996) Characterization of the csmD and csmE genes from Chlorobium tepidum. The CsmA, CsmC, CsmD, and CsmE proteins are components of the chlorosome envelope. Photosynth Res 50:41–59. doi:10.1007/BF00018220
Chung S, Frank G, Zuber H et al (1994) Genes encoding 2 chlorosome components from the green sulfur bacteria Chlorobium vibrioforme strain 83271d and Chlorobium tepidum. Photosynth Res 41:261–275. doi:10.1007/BF02184167
Chung S, Shen G, Ormerod J et al (1998) Insertional inactivation studies of the csmA and csmC genes of the green sulfur bacterium Chlorobium vibrioforme 8327: the chlorosome protein CsmA is required for viability but CsmC is dispensable. FEMS Microbiol Lett 164:353–361. doi:10.1111/j.1574-6968.1998.tb13109.x
Cogdell RJ, Gardiner AT, Roszak AW et al (2004) Rings, ellipses and horseshoes: how purple bacteria harvest solar energy. Photosynth Res 81:207–214. doi:10.1023/B:PRES.0000036883.56959.a9
DeLano WL (2002) The pymol molecular graphics system. DeLano Scientific, Palo Alto
Feick RG, Fuller RC (1984) Topography of the photosynthetic apparatus of Chloroflexus aurantiacus. Biochemistry 23:3693–3700. doi:10.1021/bi00311a019
Foidl M, Golecki JR, Oelze J (1998) Chlorosome development in Chloroflexus aurantiacus. Photosynth Res 55:109–114. doi:10.1007/BF02184154
Frigaard N-U, Bryant D (2006) Chlorosomes: antenna organelles in green photosynthetic bacteria. In: Shively JM (ed) Complex intracellular structures in prokaryotes, microbiology monographs, vol 2. Springer, Berlin
Frigaard NU, Chew AGM, Li H et al (2003) Chlorobium tepidum: insights into the structure, physiology, and metabolism of a green sulfur bacterium derived from the complete genome sequence. Photosynth Res 78:93–117. doi:10.1023/B:PRES.0000004310.96189.b4
Frigaard N-U, Sakuragi Y, Bryant DA (2004a) Gene inactivation in the cyanobacterium Synechococcus sp. PCC 7002 and the green sulfur bacterium Chlorobium tepidum using in vitro-made DNA constructs and natural transformation. In: Carpentier R (ed) Methods in molecular biology (photosynthesis research protocols). Humana Press, Totowa
Frigaard NU, Li H, Milks KJ et al (2004b) Nine mutants of Chlorobium tepidum each unable to synthesize a different chlorosome protein still assemble functional chlorosomes. J Bacteriol 186:646–653. doi:10.1128/JB.186.3.646-653.2004
Frigaard NU, Li H, Martinsson P et al (2005) Isolation and characterization of carotenosomes from a bacteriochlorophyll c-less mutant of Chlorobium tepidum. Photosynth Res 86:101–111. doi:10.1007/s11120-005-1331-8
Fuller RC (1999) Microbial inclusions with special reference to PHA inclusions and intracellular boundary envelopes. Int J Biol Macromol 25:21–29. doi:10.1016/S0141-8130(99)00011-2
Ganapathy S, Oostergetel GT, Wawrzyniak PK et al (2009) Alternating syn-anti bacteriochlorophylls form concentric helical nanotubes in chlorosomes. PNAS 106:8525–8530. doi:10.1073/pnas.0903534106
Gerola PD, Olson JM (1986) A new bacteriochlorophyll a-protein complex associated with chlorosomes of green sulfur bacteria. Biochim Biophys Acta 848:69–76. doi:10.1016/0005-2728(86)90161-1
Hohmann-Marriott MF, Blankenship RE (2007) Hypothesis on chlorosome biogenesis in green photosynthetic bacteria. FEBS Lett 581:800–803. doi:10.1016/j.febslet.2007.01.078
Holo H, Brochdue M, Ormerod JG (1985) Glycolipids and the structure of chlorosomes in green bacteria. Arch Microbiol 143:94–99. doi:10.1007/BF00414775
Holzwarth AR, Schaffner K (1994) On the structure of bacteriochlorophyll molecular aggregates in the chlorosomes of green bacteria: a molecular modeling study. Photosynth Res 41:225–233. doi:10.1007/BF02184163
Ikonen TP, Li H, Psencik J et al (2007) X-ray scattering and electron cryomicroscopy study on the effect of carotenoid biosynthesis to the structure of Chlorobium tepidum chlorosomes. Biophys J 93:620–628. doi:10.1529/biophysj.106.101444
Li H, Bryant DA (2009) Envelope proteins of the CsmB/CsmF and CsmC/CsmD motif families influence the size, shape and composition of chlorosomes in Chlorobaculum tepidum. J Bacteriol [Epub ahead of print]. doi: 10.1128/JB.00707-09
Li YF, Zhou WL, Blankenship RE et al (1997) Crystal structure of the bacteriochlorophyll a protein from Chlorobium tepidum. J Mol Biol 271:456–471. doi:10.1006/jmbi.1997.1189
Li H, Frigaard NU, Bryant DA (2006) Molecular contacts for chlorosome envelope proteins revealed by cross-linking studies with chlorosomes from Chlorobium tepidum. Biochemistry 45:9095–9103. doi:10.1021/Bi060776y
Linnanto J, Korppi-Tommopa JEI, Helenius VM (1999) Electronic states, absorption spectrum and circular dichroism spectrum of the photosynthetic bacterial LH2 antenna of Rhodopseudomonas acidophila as predicted by exciton theory and semiempirical calculations. J Phys Chem B 103:8739–8750
Manske AK, Glaeser J, Kuypers MMM et al (2005) Physiology and phylogeny of green sulfur bacteria forming a monospecific phototrophic assemblage at a depth of 100 meters in the black sea. Appl Environ Microbiol 71:8049–8060. doi:10.1128/AEM.71.12.8049-8060.2005
Matthews BW, Fenna RE, Bolognesi MC et al (1979) Structure of a bacteriochlorophyll a-protein from the green photosynthetic bacterium Prosthecochloris aestuarii. J Mol Biol 131:259–285. doi:10.1016/0022-2836(79)90076-7
Melø TB, Frigaard N-U, Matsuura K et al (2000) Electronic energy transfer involving carotenoid pigments in chlorosomes of two green bacteria: Chlorobium tepidum and Chloroflexus aurantiacus. Spectrochim Acta A 56:2001–2010. doi:10.1016/S1386-1425(00)00289-4
Milks KJ, Danielsen M, Persson S et al (2005) Chlorosome proteins studied by MALDI-TOF-MS: topology of CsmA in Chlorobium tepidum. Photosynth Res 86:113–121. doi:10.1007/s11120-005-3757-4
Montaño GA, Wu HM, Lin S et al (2003) Isolation and characterization of the B798 light-harvesting baseplate from the chlorosomes of Chloroflexus aurantiacus. Biochemistry 42:10246–10251. doi:10.1021/bi034350k
Oelze J, Golecki JR (1995) Membranes and chlorosomes of green bacteria: structure, composition and development. In: Blankenship RE, Madigan MT, Bauer CE (eds) Anoxygenic photosynthetic bacteria. Kluwer, Dordrecht, The Netherlands
Olsen JD, Sockalingum GD, Robert B et al (1994) Modification of a hydrogen-bond to a bacteriochlorophyll a molecule in the light-harvesting 1-antenna of Rhodobacter sphaeroides. PNAS 91:7124–7128
Pedersen MØ, Borch J, Højrup P et al (2006) The light-harvesting antenna of Chlorobium tepidum: interactions between the FMO protein and the major chlorosome protein CsmA studied by surface plasmon resonance. Photosynth Res 89:63–69. doi:10.1007/s11120-006-9081-9
Pedersen MØ, Pham L, Steensgaard DB et al (2008a) A reconstituted light-harvesting complex from the green sulfur bacterium Chlorobium tepidum containing CsmA and bacteriochlorophyll a. Biochemistry 47:1435–1441. doi:10.1021/Bi701616r
Pedersen MØ, Underhaug J, Dittmer J et al (2008b) The three-dimensional structure of CsmA: a small antenna protein from the green sulfur bacterium Chlorobium tepidum. FEBS Lett 582:2869–2874. doi:10.1016/j.febslet.2008.07.020
Ponder JW (2004) TINKER: software tools for molecular design. Washington University School of Medicine, Saint Louis
Psencik J, Ikonen TP, Laurinmaki P et al (2004) Lamellar organization of pigments in chlorosomes, the light harvesting complexes of green photosynthetic bacteria. Biophys J 87:1165–1172. doi:10.1529/biophysj.104.040956
Pšenčík J, Collins AM, Liljeroos L et al (2009) Structure of chlorosomes from the green filamentous bacterium Chloroflexus aurantiacus. J Bacteriol [Epub ahead of print]. doi:10.1128/JB.00690-09
Sakuragi Y, Frigaard NU, Shimada K et al (1999) Association of bacteriochlorophyll a with the CsmA protein in chlorosomes of the photosynthetic green filamentous bacterium Chloroflexus aurantiacus. BBA-Bioenergetics 1413:172–180. doi:10.1016/S0005-2728(99)00092-4
Schmidt K (1980) Comparative-study on the composition of chlorosomes (Chlorobium vesicles) and cytoplasmic membranes from Chloroflexus aurantiacus strain-ok-70-fl and Chlorobium limicola f thiosulfatophilum strain-6230. Arch Microbiol 124:21–31. doi:10.1007/BF00407024
Shibata Y, Saga Y, Tamiaki H et al (2007) Polarized fluorescence of aggregated bacteriochlorophyll c and baseplate bacteriochlorophyll a in single chlorosomes isolated from Chloroflexus aurantiacus. Biochemistry 46:7062–7068. doi:10.1021/bi0623072
Shibata Y, Saga Y, Tamiaki H et al (2009) Anisotropic distribution of emitting transition dipoles in chlorosome from Chlorobium tepidum: fluorescence polarization anisotropy study of single chlorosomes. Photosynth Res 100:67–78. doi:10.1007/s11120-009-9429-z
Sørensen PG, Cox RP, Miller M (2008) Chlorosome lipids from Chlorobium tepidum: characterization and quantification of polar lipids and wax esters. Photosynth Res 95:191–196. doi:10.1007/s11120-007-9242-5
Staehelin LA, Golecki JR, Drews G (1980) Supramolecular organization of chlorosomes (Chlorobium vesicles) and of their membrane attachment sites in Chlorobium limicola. Biochim Biophys Acta 589:30–45. doi:10.1016/0005-2728(80)90130-9
Sturgis JN, Olsen JD, Robert B et al (1997) Functions of conserved tryptophan residues of the core light-harvesting complex of Rhodobacter sphaeroides. Biochemistry 36:2772–2778. doi:10.1021/bi962524a
Tronrud DE, Schmid MF, Matthews BW (1986) Structure and X-ray amino-acid-sequence of a bacteriochlorophyll a protein from Prosthecochloris aestuarii refined at 1.9 Å resolution. J Mol Biol 188:443–454. doi:10.1016/0022-2836(86)90167-1
Tronrud DE, Wen JZ, Gay L et al (2009) The structural basis for the difference in absorbance spectra for the FMO antenna protein from various green sulfur bacteria. Photosynth Res 100:79–87. doi:10.1007/s11120-009-9430-6
van Dorssen RJ, Gerola PD, Olson JM et al (1986) Optical and structural-properties of chlorosomes of the photosynthetic green sulfur bacterium Chlorobium limicola. Biochim Biophys Acta 848:77–82. doi:10.1016/0005-2728(86)90162-3
van Noort PI, Francke C, Schoumans N et al (1994) Chlorosomes of green sulfur bacteria: pigment composition and energy-transfer. Photosynth Res 41:193–203. doi:10.1007/BF02184160
Vassilieva EV, Stirewalt VL, Jakobs CU et al (2002) Subcellular localization of chlorosome proteins in Chlorobium tepidum and characterization of three new chlorosome proteins: CsmF, CsmH, and CsmX. Biochemistry 41:4358–4370. doi:10.1021/bi012051u
Wen JZ, Zhang H, Gross ML et al (2009) Membrane orientation of the FMO antenna protein from Chlorobaculum tepidum as determined by mass spectrometry-based footprinting. PNAS 106:6134–6139. doi:10.1073/pnas.0901691106
Acknowledgments
We thank Dr R. P. Cox for critical reading of the manuscript. This study was supported by the Danish National Research Foundation by grants to NCN, NUF, and MM. Support from Academy of Finland (contract No. 123801) is acknowledged by JL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pedersen, M.Ø., Linnanto, J., Frigaard, NU. et al. A model of the protein–pigment baseplate complex in chlorosomes of photosynthetic green bacteria. Photosynth Res 104, 233–243 (2010). https://doi.org/10.1007/s11120-009-9519-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-009-9519-y