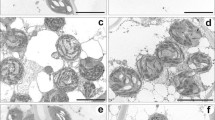
Abstract
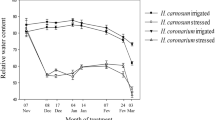
Haberlea rhodopensis is a homoiochlorophyllous desiccation-tolerant plant growing mostly in shaded rock rifts below the trees at very low light intensity. These shade plants are very sensitive to photoinhibition and do not survive desiccation at irradiance of 350 μmol m−2 s−1, whereas plants growing on the top of rocks exposed to full sunlight (sun plants) can survive at even higher light intensities regularly. The aim of the present study was to establish how acclimation to different light intensities influences the expression of selected drought-responsive genes and the physiological activity during desiccation of shade and sun plants under controlled culture conditions. The photosynthetic activity was higher in sun plants not only when fully hydrated but also during dehydration. Thus, the higher photosynthetic capacity, reflected in PSII but especially in PSI activity, is accompanied by a reduced susceptibility to photodamage. For most of the genes examined, drought was the main factor in regulation; in addition, some were light modulated like genes coding for putative superoxide dismutase (SOD), ascorbate peroxidase (APX) and thioredoxin (TRX), whereby the former was almost purely light regulated. Differences between sun and shade plants concerned mainly on the time course. Whereas some genes reacted already at moderate desiccation only in sun plants (genes for monodehydroascorbate reductase (MDAR), plastidic translocase (PTL) similar to OEP16 and one of the genes, newly annotated ELIP-like, specific for H. rhodopensis), especially a gene for a putative UDP-glucuronic acid decarboxylase (UDP) retained its enhanced expression longer during recovery. Thus, these genes are probably especially important for survival and recovery in sun plants.





Similar content being viewed by others
References
Alamillo JM, Bartels D (1996) Light and stage of development influence the expression of desiccation-induced genes in the resurrection plant Craterostigma plantagineum. Plant Cell Environ 19:300–310
Alamillo JM, Bartels D (2001) Effects of desiccation on photosynthesis pigments and the ELIP-like dsp 22 protein complexes in the resurrection plant Craterostigma plantagineum. Plant Sci 160:1161–1170
Apostolova E, Rashkova M, Anachkov N, Denev I, Toneva V, Minkov I, Yahubyan G (2012) Molecular cloning and characterization of cDNAs of the superoxide dismutase gene family in the resurrection plant Haberlea rhodopensis. Plant Physiol Biochem 55:85–92
Charuvi D, Nevo R, Shimoni E, Naveh L, Zia A, Adam Z, Farrant JM, Kirchhoffd H, Reich Z (2015) Photoprotection conferred by changes in photosynthetic protein levels and organization during dehydration of a homoiochlorophyllous resurrection plant. Plant Physiol 167:1554–1565
Chomzynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 162:156–159
Daskalova E, Dontcheva S, Yahubyan G, Minkov I, Toneva V (2011) A strategy for conservation and investigation of the protected resurrection plant Haberlea rhodopensis Friv. BioRisk 6:41–60
Demmig-Adams B, Adams WW III, Barker DH, Logan BA, Bowling DR, Verhoeven AS (1996) Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol Plant 98:253–264
Dinakar C, Bartels D (2012) Light response, oxidative stress management and nucleic acid stability in closely related Linderniaceae species differing in desiccation tolerance. Planta 236:541–555
Dinakar C, Bartels D (2013) Desiccation tolerance in resurrection plants: new insights from transcriptome, proteome and metabolome analysis. Front Plant Sci 4:482. doi:10.3389/fpls.2013.00482
Djilianov D, Ivanov S, Moyankova D, Miteva L, Kirova E, Alexieva V, Joudi M, Peshev D, Van den Ende W (2011) Sugar ratios, glutathione redox status and phenols in the resurrection species Haberlea rhodopensis and the closely related non-resurrection species Chirita eberhardtii. Plant Biol 13:767–776
Farrant JM, Bartsch S, Loffell D, van der Willigen C, Whittaker A (2003) An investigation into the effects of light on the desiccation of three resurrection plants species. Plant Cell Environ 26:1275–1286
Gechev TS, Benina M, Obata T, Tohge T, Sujeeth N, Minkov I, Hille J, Temanni M-R, Marriott AS, Bergstrӧm E, Thomas-Oates J, Antonio C, Mueller-Roeber B, Schippers JHM, Fernie AR, Toneva V (2013) Molecular mechanisms of desiccation tolerance in the resurrection glacial relic Haberlea rhodopensis. Cell Mol Life Sci 70:689–709
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and photochemical quenching of chlorophyll fluorescence. Biochem Biophys Acta 990:87–92
Georgieva K, Maslenkova L (2006) Thermostability and photostabitity of PSII in leaves of resurrection plant Haberlea rhodopensis studied by means of chlorophyll fluorescence. Z Naturforsch 61c:234–240
Georgieva K, Lenk S, Buschmann C (2008) Responses of the resurrection plant Haberlea rhodopensis to high irradiance. Photosynthetica 46:208–215
Georgieva K, Röding A, Büchel C (2009) Changes in some thylakoid membrane proteins and pigments upon desiccation of the resurrection plant Haberlea rhodopensis. J Plant Physiol 166:1520–1528
Georgieva K, Sárvári É, Keresztes Á (2010) Protection of thylakoids against combined light and drought by a lumenal substance in the resurrection plant Haberlea rhodopensis. Ann Bot 105:117–126
Kleine T, Kindgren P, Benedict C, Hendrickson L, Strand A (2007) Genome-wide gene expression analysis reveals a critical role for CRYPTOCHROME1 in the response of Arabidopsis to high irradiance. Plant Physiol 144:1391–1406
Marshall OJ (2004) PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 20:2471–2472
Mihailova G, Petkova S, Büchel C, Georgieva K (2011) Desiccation of the resurrection plant Haberlea rhodopensis at high temperature. Photosynth Res 108:5–13
Moore J, Farrant J (2012) A systems-based molecular biology analysis of resurrection plants for crop and forage improvement in arid environments. In: Tuteja N, Gill SS, Tiburcio AF, Tuteja R (eds) Improving crop resistance to abiotic stress, 1st edn. Wiley, Weinheim, pp 399–418
Morse M, Rafudeen M, Farrant JM (2011) An overview of the current understanding of desiccation tolerance in the vegetative tissues of higher plants. Adv Bot Res 57:319–347
Moyankova D, Mladenov P, Berkov S, Peshev D, Georgieva D, Djilianov D (2014) Metabolic profiling of the resurrection plant Haberlea rhodopensis during desiccation and recovery. Physiol Plant 152:675–687
Muslin EH, Homann PH (1992) Light as a hazard for the desiccation-resistant “resurrection” fern Polypodium polypoides L. Plant Cell Environ 15:81–89
Neale AD, Blomstedt CK, Bronson P, Le TN, Guthridge K, Evand D, Gaff DF, Hamill JD (2000) The isolation of genes from the resurrection grass Sporobolus stapfianus which are induced during severe drought stress. Plant Cell Environ 23:265–277
Pfaffl M (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucl Acids Res 29:e45–e45
Pohlmeyer K, Soll J, Steinkamp T, Hinnah S, Wagner R (1997) Isolation and characterization of an amino acid-selective channel protein present in the chloroplastic outer envelope membrane. PNAS 94:9504–9509
Rapparini F, Neri L, Mihailova G, Petkova S, Georgieva K (2015) Light growth environment affects the photoprotective mechanisms of the resurrection angiosperm Haberlea rhodopsensis Friv. in response to desiccation and rehydration at morphological, physiological and biochemical levels. Environ Exp Bot 113:67–79
Rodriguez MCS, Edsgärd D, Hussain SS, Alquezar D, Rasmussen M, Gilbert T, Nielsen BH, Bartels D, Mundy J (2010) Transcriptomes of the desiccation-tolerant resurrection plant Craterostigma plantagineum. Plant J 63:212–228
Seel WE, Hendry GA, Lee JA (1992) Effects of desiccation on some activated oxygen processing enzymes and antioxidants in mosses. J Exp Bot 43:1031–1037
Sherwin H, Farrant J (1998) Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscose. Plant Growth Regul 24:203–210
Terzaghi WB, Cashmore AR (1995) Light-regulated transcription. Ann Rev Plant Biol 46:445–474
Thimm O, Blaesing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37:914–939
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Xiao L, Yang G, Zhang L, Yang X, Zhao S, Ji Z, …, Xu Y (2015) The resurrection genome of Boea hygrometrica: a blueprint for survival of dehydration. PNAS 112:5833–5837
Zeng Q, Chen X, Wood AJ (2002) Two early light-inducible protein (ELIP) cDNAs from the resurrection plant Tortula ruralis are differentially expressed in response to desiccation, rehydration, salinity, and high light. J Exp Bot 53:1197–1205
Acknowledgements
We gratefully acknowledge support from the DAAD (grant number 54392417) and Bulgarian Science Fund (DNTS/Germany 01/0001).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Suppl. Fig. 1
(DOC 13579 kb)
Suppl. Fig. 2
(DOC 211 kb)
Suppl. Table 1
(DOC 43 kb)
Suppl. material NA1
(DOCX 2489 kb)
Suppl. material NA2
(DOCX 1275 kb)
Rights and permissions
About this article
Cite this article
Mihailova, G., Abakumov, D., Büchel, C. et al. Drought-Responsive Gene Expression in Sun and Shade Plants of Haberlea rhodopensis Under Controlled Environment. Plant Mol Biol Rep 35, 313–322 (2017). https://doi.org/10.1007/s11105-017-1025-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-017-1025-3