Abstract
Aims and background
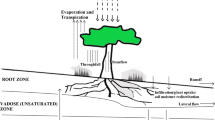
Root growth creates a gradient in age at both the scale of the single root, from distal to proximal parts, but also at the root system level when young branch roots emerge from the axis or new nodal roots are emitted that may reach same soil domain as older roots. It is known that a number of root functions will vary with root type and root tissue age (e.g. respiration, exudation, ion uptake, root hydraulic conductance, mucilage release…) and so will the resulting rhizosphere properties. The impact of the distribution of root demography with depth, and related functions, on the overall functioning of the root system is fundamental for an integration of processes at the root system scale.
Scope and conclusion
Starting from methods for measuring root demography, we discuss the availability of data related to root age and its spatial distribution, considering plant types (monocot/dicot, perennial/annuals) which may exhibit different patterns. We then give a detailed review of variation of root/rhizosphere properties related to root age, focusing on root water uptake processes. We examine the type of response of certain properties to changes in age and whether a functional relationship can be derived. Integration of changing root properties with age into modelling approaches is shown from 3D models at the single plant scale to approaches at the field scale based on integrated root system age. Functional structural modelling combined with new development in non-invasive imaging of roots show promises for integrating influence of age on root properties, from the local to whole root system scales. However, experimental quantification of these properties, such as hydraulic conductance variation with root age and root types, or impact of mucilage and its degradation products on rhizosphere hydraulic properties, presently lag behind the theoretical developments and increase in computational power.






Similar content being viewed by others
Abbreviations
- TTC assay:
-
Triphenyltetrazolium chloride assay
- MRI:
-
Magnetic resonance imaging
- X-ray CT:
-
X-ray computed tomography
- Kh:
-
Root hydraulic axial conductance
- Lr:
-
Root hydraulic radial conductivity
- Lo:
-
Root system hydraulic conductance (not normalized by root surface area)
- Lpr:
-
Root system hydraulic conductivity (normalized by root surface area)
References
Adiredjo AL, Navaud O, Grieu P, Lamaze T (2014) Hydraulic conductivity and contribution of aquaporins to water uptake in roots of four sunflower genotypes. Bot Stud 55:75
Ahmed MA, Kroener E, Holz M, Zarebanadkouki M, Carminati A (2014) Mucilage exudation facilitates root water uptake in dry soil. Funct Plant Biol. doi:10.1071/FP13330
Ahmed S, Klassen TN, Keyes S, Daly M, Jones DL, Mavrogordato M, Sinclair I, Roose T (2015) Imaging the interaction or roots and phosphate fertiliser granules using 4D X-ray tomography. Plant Soil. doi:10.1007/s11104-015-2425-5
Alm DM, Cavelier J, Nobel PS (1992) A finite-element model of radial and axial conductivities for individual roots development and validation for two desert succulents. Ann Bot 69:87–92
Arbogast T, Obeyesekere M, Wheeler MF (1993) Numerical methods for the simulation of flow in root-soil system. SIAM J Numer Anal 30:1677–1702
Aura E (1996) Modelling non-uniform soil water uptake by a single plant root. Plant Soil 186:237–243
Barraclough PB (1989) Root growth, macro-nutrient uptake dynamics and soil fertility requirements of a high-yielding winter oilseed rape crop. Plant Soil 119:59–70
Baxter I, Hosman PS, Rus A, Lahner B, Borevitz JO, Muthukuma B, Mickelbar MV, Schreiber L, Franke RB, Salt DE (2009) Root suberin forms an extracellular barrier that affects Water relations and mineral nutrition in Arabidopsis. PLoS Genet 5(5):e1000492
Bechmann M, Schneider C, Carminati A, Vetterlein D, Attinger S, Hildebrandt A (2014) Effect of parameter choice in root water uptake models – the arrangement of root hydraulic properties within the root architecture affects dynamics and efficiency of root water uptake. Hydrol Earth Syst Sci 18:4189–4206
Bouma TJ, Yanai RD, Elkin AD, Hartmond U, Flores-Alva DE, Eissenstat DM (2001) Estimating age dependent costs and benefits of roots with contrasting life span: comparing apples and oranges. New Phytol 150(3):685–695
Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN (2007) A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318:801–806
Bramley H, Turner NC, Turner DW, Tyerman SD (2009) Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiol 150(1):348–364
Brodersen CR, McElrone A (2013) Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants. Front Plant Sci 4:108
Buchard C, McCully M, Canny M (1999) Daily embolism and refilling of root xylem vessels in three dicotyledonous crop plants. Agronomie 19:97–106
Burton AL, Lynch JP, Brown KM (2013) Spatial distribution of phenotypic variation in root cortical aerenchyma of maize (Zea mays L.). Plant Soil 367:263–274
Carminati A (2013) Rhizosphere wettability decreases with root age: a problem or a strategy to increase water uptake of young roots? Front Plant Sci 4:298
Carminati A, Vetterlein D (2013) Plasticity of rhizosphere hydraulic properties as a key for efficient utilization of scarce resources. Ann Bot 112(2):277–290
Carminati A, Vetterlein D, Koebernick N, Blaser S, Weller U, Vogel HJ (2013) Do roots mind the gap? Plant Soil 367(1–2):651–661
Choat B, Lahr EC, Melcher PJ, Zwieniecki MA, Holbrook NM (2005) The spatial pattern of air seeding thresholds in mature sugar maple trees. Plant Cell Environ 28(9):1082–1089
Clarkson DT, Carvajal M, Henzler T, Waterhouse RN, Smyth AJ, Cooke DT, Steudle E (2000) Root hydraulic conductance: diurnal aquaporin expression and the effects of nutrient stress. J Exp Bot 51:61–70
Comas LH, Eissenstat DM, Lakso AN (2000) Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol 147:171–178
Couvreur V, Vanderborght J, Draye X, Javaux M (2014) Dynamic aspects of soil water availability for isohydric plants: focus on root hydraulic resistances. Water Ressour Res. doi:10.1002/2014WR015608
Cushman JH (1984) Numerical study of some age-dependent parameters in root nutrient uptake. Plant Soil 79:123–141
Deacon JW (1992) Current issues in rhizosphere biology with special reference to cereals. In: Munck L (ed) Barley genetics VI. Volume II. Barley research reviews 1986-91, Session and workshop summaries. Proceedings of the sixth international barley genetics symposium 1991. Munksgaard International Publishers Ltd, Helsingborg
Domec JC, Scholz FG, Bucci SJ, Meinzer FC, Goldstein G, Villalobos-Vega R (2006) Diurnal and seasonal variation in root xylem embolism in neotropical savanna woody species: impact on stomatal control of plant water status. Plant Cell Environ 29:26–35
Doussan C, Pagès L, Vercambre G (1998a) Modelling of the hydraulic architecture of root systems: an integrated approach to water absorption - model description. Ann Bot 81(2):213–223
Doussan C, Vercambre G, Pagès L (1998b) Modelling of the hydraulic architecture of root systems: An integrated approach to water absorption - distribution of axial and radial conductances in maize. Ann Bot 81(2):225–232
Doussan C, Vercambre G, Pagès L (1999) Water uptake by two contrasting root systems (maize, peach tree): results from a model of hydraulic architecture. Agronomie 19:255–263
Doussan C, Pierret A, Garrigues E, Pagès L (2006) Water uptake by plant roots: II–modelling of water transfer in the soil root-system with explicit account of flow within the root system–comparison with experiments. Plant Soil 283(1–2):99–117
Draye X, Kim Y, Lobet G, Javaux M (2010) Model-assisted integration of physiological and environmental constraints affecting the dynamic and spatial patterns of root water uptake from soils. J Exp Bot 61(8):2145–2155
Dubach M, Russelle MP (1995) Reducing the cost of estimating root turnover with horizontally installed minirhizotrons. Agron J 87(2):258–263
Dunbabin VM, Diggle AJ, Rengel Z, van Hugten R (2002) Modelling the interactions between water and nutrient uptake and root growth. Plant Soil 239(1):19–38
Dunbabin VM, Postma JA, Schnepf A, Pagès L, Javaux M, Wu L, Leitner D, Chen YL, Rengel Z, Diggle AJ (2013) Modelling root–soil interactions using three–dimensional models of root growth, architecture and function. Plant Soil 372(1–2):93–124
Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147:33–42
Feddes RA, Kowalik PJ, Zaradny H (1978) Simulation of field water use and crop yield Simul. Monogr. PUDOC, Wageningen
Fischer MCT, Eissenstat DM, Lynch JP (2002) Lack of evidence for programmed root senescence in common bean (Phaseolus vulgaris) grown at different levels of phosphorus supply. New Phytol 153:63–71
Forbes PJ, Black KE, Hooker JE (1997) Temperature-induced alteration to root longevity in lolium perenne. Plant Soil 190(1):87–90
Frensch J, Steudle E (1989) Axial and radial hydraulic resistance to roots of maize (Zea mays L.). Plant Physiol 91:719–726
Froux F, Ducrey M, Dreyer E, Huc R (2005) Vulnerability to embolism differs in roots and shoots and among three Mediterranean conifers: consequences for stomatal regulation of water loss? Trees-Structure and Function 19:137–144
Gambetta GA, Fei J, Rost TL, Knipfer T, Matthews MA, Shackel KA, Walker MA, McElrone AJ (2013) Water uptake along the length of grapevine fine roots: developmental anatomy, tissue-specific aquaporin expression, and pathways of Water transport. Plant Physiol 163:1254–1265
Gao S, Pan WL, Koenig RT (1998) Integrated root system age in relation to plant nutrient uptake activity. Agron J 90:505–510
Gardner WR (1960) Dynamic aspects of water availability to plants. Soil Sci 89(2):63–73
Hajek P, Hertel D, Leuschner C (2014) Root order-and root age-dependent response of two poplar species to belowground competition. Plant Soil 377(1–2):337–355
Heinen M (2001) FUSSIM2: brief description of the simulation model and application to fertigation scenarios. Agronomie 21:285–296
Herkerlrath WN, Miller EE, Gardner WR (1977) Water uptake by plants. 1- divided root experiments. Soil Sci Soc Am J 41:1033–1038
Hillel D, Talpaz H, Van Keulen H (1976) A macroscopic-scale model of water uptake by a nonuniform root system and of water and salt movement in the soil profile. Soil Sci 121:242–255
Hukin D, Cochard H, Dreyer E, Le Thiec D, Bogeat-Triboulot MB (2005) Cavitation vulnerability in roots and shoots: does Populus euphratica Oliv., a poplar from arid areas of Central Asia, differ from other poplar species. J Exp Bot 56:2003–2010
Javaux M, Schröder T, Vanderborght J, Vereecken H (2008) Use of a three–dimensional detailed modeling approach for predicting root water uptake. Vadose Zone J 7:1079–1088
Johnson DM, Sherrard ME, Domec JC, Jackson RB (2014) Role of aquaporin activity in regulating deep and shallow root hydraulic conductance during extreme drought. Trees 28:1323–1331
Knipfer T, Besse M, Verdeil JL, Fricke W (2011) Aquaporin-facilitated water uptake in barley (Hordeum vulgare L.) roots. J Exp Bot 62:4115–4126
Koebernick N, Weller U, Huber K, Schlüter S, Vogel HJ, Jahn R, Vereecken H, Vetterlein D (2014) In situ visualization and quantification of three-dimensional root system architecture and growth using X-Ray computed tomography. Vadose Zone J 13(8). doi:10.2136/vzj2014.03.0024
Koebernick N, Huber K, Kerkhofs E, Vanderborght J, Javaux M, Vereecken H, Vetterlein D (2015) Unraveling the hydrodynamics of split root water uptake experiments using CT scanned root architectures and three dimensional flow simulations. Front Plant Sci 6:370
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press Pub, 495 p
Kroener E, Zarebanadkouki M, Kaestner A, Carminati A (2014) Nonequilibrium water dynamics in the rhizosphere: how mucilage affects water flow in soils. Water Resour Res 50:6479–6495
Kroener E, Ahmed MA, Carminati A (2015) Roots at the percolation threshold. Phys Rev E 91(4):042706
Laur J, Hacke UG (2013) Transpirational demand affects aquaporin expression in poplar roots. J Exp Bot 64(8):2283–2293
Leitner D, Meunier F, Bodner G, Javaux M, Schnepf A (2014) Impact of contrasted maize root traits at flowering on water stress tolerance – A simulation study. Field Crop Res 165:127–137
Liljeroth E (1995) Comparisons of early root cortical senescence between barley cultivars, Triticum species and other cereals. New Phytol 130(4):495–501
Lobet G, Couvreur V, Meunier F, Javaux M, Draye X (2014) Plant Water uptake in drying soils. Plant Physiol 164:1619–1627
Lopez FB, Nobel PS (1991) Root hydraulic conductivity of two cactus species in relation to root age, temperature, and soil water status. J Exp Bot 42(2):143–149
Malagoli P, Le Deunff E (2014) An updated model for nitrate uptake modelling in plants. II. assessment of active root involvement in nitrate uptake based on integrated root system age: measured versus modelled outputs. Ann Bot 113:1007–1019
McCully M (1999) Root xylem embolisms and refilling. relation to water potentials of soil, roots, and leaves, and osmotic potentials of root xylem sap. Plant Physiol 119:1001–1008
McCully ME, Boyer JS (1997) The expansion of maize root-cap mucilage during hydration. 3. changes in water potential and water content. Physiol Plant 99:169–177
McElrone AJ, Pockman WT, Martínez-Vilalta J, Jackson RB (2004) Variation in xylem structure and function in stems and roots of trees to 20 m depth. New Phytol 163(3):507–517
Miki NK, Clarke KJ, McCully EM (1980) A histological and histochemical comparison of the mucilages on the root tips of several grasses. Can J Bot 58:2581–2595
Mimmo T, Marzadori C, Francioso O, Deiana S, Gessa CE (2003) Effects of aluminium soption on calcium-polygalacturonate network used as soil-root interface model. Biopolymers 70:655–661
Molz FJ (1981) Models of water transport in the soil-plant system: a review. Water Resour Res 17:1245–1260
Mooney SJ, Pridmore TP, Helliwell J, Bennett MJ (2012) Developing X-ray computed tomography to non-invasively image 3-D root systems architecture in soil. Plant Soil 352(1–2):1–22
Moradi AB, Carminati A, Vetterlein D, Vontobel P, Lehmann E, Weller U, Hopmans JW, Vogel H-J, Oswald SE (2011) Three-dimensional visualization and quantification of water content in the rhizosphere. New Phytol 192(3):653–663
North GB, Nobel PS (1996) Radial hydraulic conductivity of individual root tissues of Opuntia ficus-indica (L) Miller as soil moisture varies. Ann Bot 77:133–142
North GB, Nobel PS (2000) Heterogeneity in Water availability alters cellular development and hydraulic conductivity along roots of a desert succulent. Ann Bot 85:247–255
Pagès L, Asseng S, Pellerin S, Diggle A (2000) Modelling root system growth and architecture. In: Smit L, AG B, Engels C, van Noordwijj M, Pellerin S, de Geijn v (eds) Root methods: a handbook. Springer Pub, pp. 113–146
Pagès L, Becel C, Boukcim H, Moreau D, Nguyen C, Voisin AS (2014) Calibration and evaluation of ArchiSimple, a simple model of root system architecture. Ecol Model 290:76–84
Palta JA, Nobel PS (1989) Influences of water status, temperature, and root age on daily patterns of root respiration for two cactus species. Ann Bot 63(6):651–662
Pierret A, Doussan C, Pagès L (2006) Spatio-temporal variations in axial conductance of primary and first-order lateral roots of a maize crop as predicted by a model of the hydraulic architecture of root systems. Plant Soil 282:117–126
Postma JA, Lynch JP (2011) Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Ann Bot 107:829–841
Pratt RB, MacKinnon ED, Venturas MD, Crous CJ, Jacobsen AL (2015) Root resistance to cavitation is accurately measured using a centrifuge technique. Tree Physiol 35:185–196
Prieto I, Rye RJ (2014) Internal hydraulic redistribution prevents the loss of root conductivity during drought. Tree Physiol 34:39–48
Ranathunge K, Schreiber L (2011) Water and solute permeabilities of Arabidopsis roots in relation to the amount and composition of aliphatic suberin. J Exp Bot 62:1961–1974
Ranathunge K, Steudle E, Lafitte R (2003) Control of water uptake by rice (Oryza sativa L.): role of the outer part of the root. Planta 217:193–205
Read DB, Bengough AG, Gregory PJ, Crawford JW, Robinson D, Scrimgeour CM, Young IM, Zhang K, Zhang X (2003) Plant roots release phospholipid surfactants that modify the physical and chemical properties of soil. New Phytol 157:315–326
Rewald B, Ephrath JE, Rachmilevitch S (2011) A root is a root is a root? Water uptake rates of citrus root orders. Plant Cell Environ 34(1):33–42
Rieger M, Litvin P (1999) Root system hydraulic conductivity in species with contrasting root anatomy. J Exp Bot 50:201–209
Schneider CL, Attinger S, Delfs JO, Hildebrandt A (2010) Implementing small scale processes at the soil-plant interface – the role of root architectures for calculating root water uptake profiles. Hydrol Earth Syst Sci 14:279–289
Schnepf A, Leitner D, Klepsch S (2012) Modeling phosphorus uptake by a growing and exuding root system. Vadose Zone J 11(3). doi:10.2136/vzj2012.0001
Somma F, Hopmans JW, Clausnitzer V (1998) Transient three-dimensional modeling of soil water and solute transport with simultaneous root growth, root water and nutrient uptake. Plant Soil 202(2):281–293
Spaeth SC, Cortes PM (1995) Root cortex death and subsequent initiation and growth of lateral roots from bare steles of chickpea. Can J Bot 73:253–261
Sperry JS, Ikeda T (1997) Xylem cavitation in roots and stems of Douglas-fir and white fir. Tree Physiol 17(4):275–280
Sperry JS, Adler FR, Campbell GS, Comstock JP (1998) Limitation of plant water use by rhizosphere and xylem conductance: results from a model. Plant Cell Environ 21:347–359
Steudle E, Peterson C (1998) How does water get through roots? J Exp Bot 49:775–788
Stingaciu L, Schulz H, Pohlmeier A, Behnke S, Zilken H, Javaux M, Vereecken H (2013) In situ root system architecture extraction from magnetic resonance imaging for water uptake modeling. Vadose Zone J 12(1). doi:10.2136/vzj2012.0019
Sutka M, Li G, Boudet J, Boursiac Y, Doumas P, Maurel C (2011) Natural variation of root hydraulics in Arabidopsis Grown in Normal and Salt-Stressed Conditions. Plant Physiol 155:1264–1276
Taleisinik E, Peyran G, Cordoba A, Arias C (1999) Water retention capacity in root segments differing in the degree of exodermis development. Ann Bot 83:19–27
Tracy SR, Black CR, Roberts JA, Sturrock C, Mairhofer S, Craigon J, Mooney SJ (2012) Quantifying the effect of soil compaction on three varieties of wheat (Triticum aestivum L.) using X-ray micro computed tomography (CT). Plant Soil 353:195–208
Tyree MT, Sperry JS (1989) Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Phys Mol Bio 40:19–13
Tyree MT, Velez V, Dalling JW (1998) Growth dynamics of root and shoot hydraulic conductance in seedlings of five neotropical tree species: scaling to show possible adaptation to differing light regimes. Oecologia 114:293–298
Van den Berg M, Driessen PM (2002) Water uptake in crop growth models for land use systems analysis I. A review of approaches and their pedigrees. Agric Ecosyst Environ 92:21–36
Varney GT, Canny MJ (1993) Rates of water uptake into the mature root system of maize plants. New Phytol 123:775–786
Vercambre G, Doussan C, Pagès L, Habib R, Pierret A (2002) Influence of xylem development on axial hydraulic conductance within Prunus root systems. Trees-Structure and Function 16:479–487
Vercambre G, Pagès L, Doussan C, Habib R (2003) Architectural analysis and synthesis of the plum tree root system in an orchard using a quantitative modelling approach. Plant Soil 251:1–11
Wang E, Smith CJ (2004) Modelling the growth and water uptake function of plant root systems: a review. Aust J Agric Res 55:501–523
Wang Z, Burch WH, Mou P, Jones RH, Mitchell RJ (1995) Accuracy of visible and ultraviolet light for estimating live root proportions with minirhizotrons. Ecology 76:2330–2334
Warren JM, Hanson PJ, Iversen CM, Kumar J, Walker AP, Wullschleger SD (2014) Root structural and functional dynamics in terrestrial biosphere models – evaluation and recommendations. New Phytol 205:59–78
Watt M, Magee LJ, McCully ME (2008) Types, structure and potential for axial water flow in the deepest roots of field-grown cereals. New Phytol 178:135–146
Wells CE, Eissenstat DM (2003) Beyond the roots of young seedlings: the influence of age and order on fine root physiology. J Plant Growth Regul 21(4):324–334
Wells CE, Glenn DM, Eissenstat DM (2002) Changes in the risk of fine-root mortality with age: a case study in peach, Prunus persica (Rosaceae). Am J Bot 89(1):79–87
Wu H, Jaeger M, Wang M, Li B, Zhang BG (2011) Three-dimensional distribution of vessels, passage cells and lateral roots along the root axis of winter wheat (Triticum aestivum). Ann Bot 107:843–853
Zappala S, Helliwell JR, Tracy SR, Mairhofer S, Sturrock CJ, Pridmore T, Bennett M, Mooney SJ (2013) Effects of X-ray dose on rhizosphere studies using X-ray computed tomography. PLoS one 8(6):E67250
Zimmermann MH (1983) Xylem structure and the ascent of sap. Springer series in wood science, Springer Verlag Pub, 143 p
Acknowledgments
Research is funded by DFG “Mucilage: the hydraulic bridge between roots and soil” VE 229/3-1 AOBJ: 610452 and by a grant from EU-EURoot project (KBBE-2011-5-289300). We thank Nico Koebernick and Katrin Huber for providing Fig. 2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric J.W. Visser.
Rights and permissions
About this article
Cite this article
Vetterlein, D., Doussan, C. Root age distribution: how does it matter in plant processes? A focus on water uptake. Plant Soil 407, 145–160 (2016). https://doi.org/10.1007/s11104-016-2849-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2849-6