Abstract
Aims
The litter decomposition effects of N deposition in combination with management practices are still unclear. The aims of this study were to test the individual and interactive effects of the two factors on Moso bamboo leaf litter decomposition.
Methods
The experiment was conducted in Moso bamboo forests over a 24-month period and included three N addition treatments (low N, 30 kg N ha−1 yr−1;medium N, 60 kg N ha−1 yr−1; and high N, 90 kg N ha−1 yr−1) and two management practices (conventional management and intensive management).
Results
The low-N treatment significantly increased the annual decomposition rate, whereas the high-N treatment significantly decreased the annual decomposition rate (P < 0.05). Intensive management alone did not significantly influence decomposition, but it amplified both the positive effects of the low-N treatment and the negative effects of the high-N treatment on litter decomposition. Intensive management weakened the positive effects of N addition on N loss but enhanced the positive effects on P loss during the early stage.
Conclusions
The effects of increasing N deposition in a plantation ecosystem were altered by the management practices, and these changes should be considered when estimating the biological effects of N deposition at regional and global scales.
Similar content being viewed by others
Introduction
Atmospheric nitrogen (N) deposition is an important driver of global environmental change. Reactive N production has increased from approximately 15 Tg N in 1860 to 187 Tg N yr−1 in 2005 and is expected to continue to increase by50–100 % by 2030 (relative to 2000 figures) as a result of human activity (Galloway et al. 2008; Reay et al. 2008). The average bulk deposition of N has increased from 13.2 kg N ha−1 yr−1 in the 1980s to 21.1 kg N ha−1 yr−1 in the 2000s across China (Liu et al. 2013). Subtropical China, with a maximum annual N deposition rate of 63.53 kg N ha−1 yr−1 (Lü and Tian 2007),is predicted to become the region with the greatest N deposition in the world by 2030 (Reay et al. 2008).
Nitrogen deposition can profoundly influence litter decomposition, a crucial ecosystem process controlling the cycling of nutrients and carbon. Nitrogen deposition may either positively, neutrally, or negatively affect litter decomposition by altering soil N availability and soil microbial decomposers (Micks et al. 2004; Knorr et al. 2005; Fang et al. 2007; Hobbie et al. 2006; Keeler et al. 2009). However, forest management practices in plantations, such as thinning, ploughing, and fertilization, can also impact litter decomposition by altering the physicochemical and biological properties of the soil (Balesdent et al. 1998; Xu et al. 1998; Pandey et al. 2007). However, little information is available regarding the interactive effects of N deposition and management practices on litter decomposition and subsequent nutrient release.
Because of a rapid growth rate and a high annual re-growth rate after harvesting, Moso bamboo (Phyllostachys heterocycla (Carr.) Mitford cv. Pubescens) forests are highly representative of plantation forests in the subtropical region of China. Moso bamboo forests are the most important source of non-wood forest products in the current China, covering an area of 3.87 million ha, representing 70 % of the country’s bamboo forested area and 80 % of the global distribution of P. pubescens(Li and Lei 2010; Song et al. 2011). Due to its enormous ecological and socioeconomic benefits and sustainable forest development policies, Moso bamboo forests have expanded rapidly in subtropical China, with an annual increase rate of 3 % in recent decades (Song et al. 2011).
To maintain high productivity in Moso bamboo plantations and achieve the maximal economic benefits, increasing numbers of farmers are adopting intensive management practices, such as the regular removal of understory vegetation, ploughing, and fertilization (Liu et al. 2011; Song et al. 2011). Typically, conventional management regularly harvests bamboo stems and shoots, without any of the other management practices mentioned above (Liu et al. 2011). The intensive management practices increase bamboo production but reduce soil microbial functional diversity (Xu et al. 2008; Zhou et al. 2009), accelerate soil organic carbon mineralization (Zhou et al. 2006; Jiang et al. 2009), and may influence litter decomposition. Litter decomposition is controlled by multiple factors. Our previous studies found that the interactive effects of N deposition and UV-B radiation on litter decomposition far exceed the effects of each factor individually (Song et al. 2014a,b). The area of Moso bamboo plantations is the region with greatest N deposition in China, both currently and in future predictions. The effects of N deposition on litter decomposition and nutrient turnover, both solely and combined with management practices, still remain unknown, limiting our understanding of the responses of Moso bamboo plantations to global environmental changes and human management practices.
In this study, a 24-month field-based experiment was conducted to test the following hypotheses: (1) N deposition accelerates P. pubescens litter decomposition; (2) intensive management practices also accelerate P. pubescens litter decomposition; and (3) the combination of N deposition and management practices has stronger effects on litter decomposition than each practice separately.
Materials and methods
Study site
The study site was located in Qingshan Town, Lin’an city (30°14′N, 119°42′E), Zhejiang Province, China. The area has a monsoonal subtropical climate with four distinct seasons. The mean annual precipitation is 1420 mm, and the mean annual temperature is 15.6 °C, with maximum and minimum temperatures of 41.7 °C and-13.3 °C, respectively. The area receives an average of approximately 1847 h of sunshine per year and features an average of 230 frost-free days per year. The monthly mean air temperatures and precipitation quantities during the study period are shown in Fig. 1.
The conventionally managed Moso bamboo plantations (CM) were originally established in the late 1970s from native evergreen broadleaf forest in sites of similar topography (southwest slope of approximately 6°) and soil type. The soils are classified as Ferrisols derived from granite (Liu et al. 2011). The intensive management practices, including annual fertilization, ploughing, and weeding with herbicide, have been conducted since 2001. In September of each year, fertilizers are distributed and followed with a deep plough to 0.3 m. Urea (450 kg ha−1), single super phosphate (450 kg ha−1) and potassium chloride (150 kg ha−1) were applied annually before 2010, and nitrate of S-based compound fertilizer (N-P2O5-K2O: 15-6-20 %, 450 kg ha−1) has been applied annually since 2010, which is equivalent to the annual addition of 67.5 kg N, 11.8 kg P, and 74.7 kg K per hectare. With the exception of bamboo stems and shoots being selectively and regularly harvested as required, the conventionally managed Moso bamboo plantations did not experience any other management practices. In the CM plantations, 32 understory species, dominated by Loropetalum chinensis (R. Br.) Oliv., Cyclobalanopsis glauca (Thunb.) Oerst., and Vaccinium bracteatum Thunb., cover 80 % of the forest floor, have a mean height of 0.7 m, and form a total shrub and herbal biomass of 2183 kg ha−1. In the intensively managed Moso bamboo plantations (IM), 11 understory species, dominated by Viola prionantha, achieve a mean height of 0.1 m, a forest floor coverage of 5 %, and a total herbal biomass of 14.6 kg ha−1.
Experimental design and measurement
In November 2012, 24 measurement plots of 20 × 20 m were established, with 12 in both the CM plantation area and the IM plantation area. The initial stand and soil characteristics of both the CM and IM are summarized in Table 1. Briefly, compared with CM, IM had significantly lower soil organic C (SOC) but higher soil total N (TN) and available nitrogen (AN). Each plot was surrounded by a 20 m-wide buffer zone to avoid disturbing nearby plots. Three N addition treatments (low-N, medium-N, high-N) and a control (no added N) with three replicate plots per treatment were established randomly in each Moso bamboo plantation management type. According to the widely used method in the previous studies simulating N deposition (Mo et al. 2006; Fang et al. 2007) and the local N deposition of 30.9 kg N ha−1 yr−1(Xie et al. 2008), the N addition treatments were conducted with a low-N addition of 30 kg N ha−1 yr−1 (N30), a medium-N addition of 60 kg N ha−1 yr−1(N60), and a high-N addition of 90 kg N ha−1 yr−1(N90). Starting in January 2013, quantitative NH4NO3 was weighed, mixed with 10 L of water and sprayed evenly onto the forest floor of each plot using an electric sprayer at the beginning of every month for a total of 12 equal applications over the entire year throughout the experiment period. These water-based additions amounted to an increase of 0.3 mm of rainfall per year (Mo et al. 2006; Fang et al. 2007). Each control treatment plot received 10 L of N-free water to control for the effects of the added water.
Leaf litter collection, decomposition and chemical analysis
In June 2012, freshly fallen leaf litter of P. pubescens was collected from the CM plantation’s floor. The samples were air-dried for a period of several months in a laboratory. Subsamples of the air-dried samples were oven-dried at 65 °C to a constant weight to determine the water content of the air-dried samples and were then ground for initial litter chemistry analysis using the methods described below.
The litterbag method was used to determine the rate of leaf litter decomposition. The litterbags were 15 × 15 cm in size and constructed from polypropylene fabric with a 0.5 × 1.0 mm mesh-size. Each litterbag was filled with 10 g of air-dried leaf litter. The starting weight of air-dried litter was converted to oven-dried weight according to the water content of air-dried samples. In December 2012, a total of 360 litterbags were positioned on the surface of the organic horizon in the 24 treatment plots. Each plot contained 15 litterbags that were divided into three equal groups, with each group including 5 litterbags for five sampling events. Three litterbags were retrieved from each plot after periods of 4, 8, 12, 18, and 24 months. After collection, any adhering soil particles were removed by gentle washing and brushing. The litter was then oven-dried at 65 °C to a constant weight and weighed. The three samples from each plot per sampling event were averaged as the value per plot. Differences between the mass at the start of the experiment and the mass at each sampling time were used to calculate the rate of leaf litter decomposition.
Oven-dried litter was ground with a grinder (DFT-50A, Wenling LINDA Machinery Co. Ltd., China). The total carbon (C) and N values were determined using a Sumigraph NC-80 high-sensitivity CN analyzer (Shimadzu, Japan). The phosphorus (P) concentration was determined using a modified Kjeldahl method followed by photometric analysis (Xuluc-Tolosa et al. 2003). The lignin concentration was determined using acid-detergent fiber methods (Rowland and Roberts 1994; Song et al. 2014a).
Data and statistical analysis
The remaining dry weight of the litter in each retrieved litterbag was expressed as a percentage of the initial litter dry weight of each litterbag. The first-order exponential decay model (Olson 1963) of the form Xt/X0 = e -kt was fitted to the decomposition data, where Xt is the net oven-dry weight remaining at time t; X0isthe initial oven-dry weight; and k is the annual decomposition rate constant (yr−1).
The nutrient release via litter decomposition was expressed as a percentage of the initial nutrient content, which was calculated by determining the nutrient content at each sampling time and dividing it by the initial nutrient content (Pancotto et al. 2003; Brandt et al. 2010): E = [(Mt × Ct)/(M0 × C0)] × 100, where E is the nutrient release (%); M0 is the initial oven-dry mass (g); C0 is the initial nutrient concentration (mg g−1); Mt is the oven-dry mass at time t; and Ct is the nutrient concentration at time t.
One-way analysis of variance (ANOVA) and least significant difference (LSD) tests were used to determine the statistical significance of differences between the annual decomposition rate, litter mass loss, and nutrient release of each sampling event among the four experimental treatments in the two plantation management types. A two-way ANOVA method was used to test the interaction of N deposition and management practices on annual litter decomposition rates. These analyses were conducted using SPSS (Statistical Package for the Social Sciences) 18.0 for Windows (SPSS Inc., Chicago, Illinois).
Results
Litter mass loss and annual decomposition rate
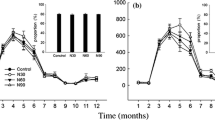
In the CM plots (Fig. 2, Table 2), both the litter mass loss and the annual decomposition rate were fastest under the N30 treatment and slowest under the N90 treatment. No significant difference in litter mass loss or annual decomposition rate was observed between the control and N60 treatments (P > 0.05). In the IM plots, the litter mass loss under the four treatments was similar to that in the CM plots but tended to slow after 18 months of decomposition (Fig. 2). Similar to the CM plots, the decomposition rates in the IM plots also showed the same trends, with the highest decomposition rate under the N30 treatment and the lowest under the N90 treatment (Table 2).
Mean (n = 3) with standard deviation of remaining leaf litter mass, as a percentage of the initial mass, during a 24-month leaf litter decomposition experiment of Phyllostachys pubescens under four treatments: low-N addition (N30, 30 kg N ha−1 yr−1), medium-N addition (N60, 60 kg N ha−1 yr−1), high-N addition (N90, 90 kg N ha−1 yr−1), and a control in Moso bamboo forest with conventional management (CM) and intensive management (IM)
The litter in the IM plots decomposed faster than in the CM plots under both the control and N30 treatments but slower under both the N60 and N90 treatments. Under the N30 and N90 treatments, this difference was significant (P < 0.05). A two-way ANOVA showed that N addition alone and N addition combined with management practices significantly affected the litter decomposition rates (P < 0.01) (Table 3).
N and P loss dynamics
In the CM plots (Fig. 3), N exhibited a fast net loss in the early 8 months of decomposition, with the fastest rate under the N60 treatment and the slowest rate under the control. The N net loss tended to slow after 12 months, and this deceleration occurred more rapidly under the N60 and N90 treatment than under the control and N30 treatments. In the IM plots, rapid N net loss also occurred in the early 8 months under all four treatments and also slowed after 12 months, with the fastest loss under the control and the slowest under the N90 treatment.
Mean litter nitrogen (N,% of initial) with the standard deviation over time for the leaf litter decomposition of Phyllostachys pubescens under four treatments: low-N addition (N30, 30 kg N ha−1 yr−1), medium-N addition (N60, 60 kg N ha−1 yr−1), high-N addition (N90, 90 kg N ha−1 yr−1), and a control in Moso bamboo forest with conventional management (CM) and intensive management (IM)
The P loss exhibited similar dynamics in both the CM and IM plots with a fast net loss in the early 8 months of decomposition that tended to slow after 8 months and especially after 18 months. P loss was fastest under the N30 treatment, followed by the control, and the slowest under the N90 treatment (Fig. 4).
Mean litter phosphorus (P,% of initial) with the standard deviation over time in the leaf litter decomposition of Phyllostachys pubescens under four treatments: low-N addition (N30, 30 kg N ha−1 yr−1), medium-N addition (N60, 60 kg N ha−1 yr−1), high-N addition (N90, 90 kg N ha−1 yr−1), and a control in Moso bamboo forest with conventional management (CM) and intensive management (IM)
Lignin degradation
Compared to N and P loss, lignin degradation was slow and did not show a significant difference between treatments after the first 4 months of decomposition in both the CM and IM plots (P > 0.05). Lignin degradation became rapid after 4 months and tended to slow again after 18 months (Fig. 5). The fastest and slowest degradation rates occurred under the N30 and N90 treatments, respectively, in both the CM and IM plots, but lignin degradation did not feature a significant difference between the N60 and control treatments throughout the entire experiment (P > 0.05).
Mean litter lignin (lignin,% of initial) with the standard deviation over time in the leaf litter decomposition of Phyllostachys pubescens under four treatments: low-N addition (N30, 30 kg N ha−1 yr−1), medium-N addition (N60, 60 kg N ha−1 yr−1), high-N addition (N90, 90 kg N ha−1 yr−1), and a control in Moso bamboo forest with conventional management (CM) and intensive management (IM)
Discussion
Effects of N deposition and management practices on litter decomposition
In the CM plots, the N30 treatment significantly increased the annual litter decomposition rate, but the N90 treatment significantly decreased the decomposition rate, and the N60 treatment did not significantly influence the decomposition rate (Table 2), which to some extent supports our first hypothesis. In a previous field-based experiment investigating the interactive effects of UV-B radiation and N deposition on litter decomposition over a 20-month period, we also observed that low-N additions (30 kg N ha−1 yr−1) significantly accelerated litter decomposition of P. pubescens (Song et al. 2014a). Nitrogen addition can enhance N availability and soil enzyme activity, thereby accelerating litter decomposition (Knorr et al. 2005; Manning et al. 2008). Lignin is a key factor in the regulation of litter decomposition, especially during the later decay stage (Austin and Ballaré 2010). Excessive N inputs have been demonstrated to suppress ligninolytic enzyme activity in the long term and correspondingly reduce litter decomposition (Fog 1988; Carreiro et al. 2000; Thirukkumaran and Parkinson 2000; Hobbie et al. 2012), although a few studies have found no evidence of negative effects (Hobbie et al. 2006). In the present study, lignin degradation was faster under the N30 treatment and slower under the N90 treatment than the control and slowed after 18 months of decomposition under all four treatments (Fig. 5). Therefore, the low-N addition facilitated lignin degradation, whereas the high-N addition suppressed lignin degradation. Our findings suggest that certain rates of N deposition, such as30 kg N ha−1 yr−1, can accelerate leaf litter decomposition of P. pubescens, whereas excessive N deposition, such as 90 kg N ha−1 yr−1, suppresses decomposition. We predict that the critical threshold of N addition is likely between 60 and 90 kg N ha−1 yr−1 for P. pubescens leaf litter decomposition.
No significant differences in the litter decomposition rate were observed between the IM and the CM plots under the control treatment (Table 2). A two-way ANOVA also showed management practices alone did not significantly affect litter decomposition (Table 3), which does not support our second hypothesis. Balesdent et al. (1998) reported that intensive management practices, such as fertilizer application and tillage, could enhance microbial activity and consequently promote soil organic C mineralization. Xu et al. (1998) also observed that intensive management practices enhanced soil enzyme activity in the Moso bamboo plantations. Our previous studies at this site also found that intensive management practices improved the activity of soil β-fructofuranosidase and cellulose (Xuan et al. 2014). Our results indicate that these positive effects on soil biological properties were still not enough to create a significant difference in litter decomposition, although they contributed to a higher decomposition rate in the IM plots than the CM plots without N addition.
A two-way ANOVA showed that management practices combined with N addition significantly influenced litter decomposition (Table 3). Moreover, when combined with a low-N addition of 30 kg N ha−1 yr−1, the management practices significantly accelerated lignin degradation and litter decay. However, when combined with a high-N addition of 90 kg N ha−1 yr−1, which significantly retarded litter decomposition, these management practices significantly decreased lignin degradation and litter decay even further (Table 2, Figs. 2 and 5). Thus, the management practices amplified both the positive effects of the low-N addition and the negative effects of the high-N addition on P. pubescens leaf litter decomposition. This pattern supports the third hypothesis, i.e., a combination of management practices and N deposition has stronger effects on litter decomposition than either factor separately.
Effects of N deposition and management practices on litter nutrient loss
The accumulation or release of nutrients from decomposing litter is predominantly regulated by the initial N content and the stoichiometric requirements of the microbial decomposers (Xu and Hirata 2005; Parton et al. 2007; Manzoni et al. 2010). N release takes place generally when the initial N content is between 0.6 and 2.8 % (Berg and Staff 1981) or the C:N ratio drops below a critical threshold of 5 to 15 (Parton et al. 2007; Manzoni et al. 2008). However, decomposers may become adapted to substrates with low N concentration by decreasing their C use efficiency and thus elevating the required critical C/N ratios of the litter for net mineralization to occur (Manzoni et al. 2008). In the current study, the initial litter N content (1.13 %) (Table 4) was in the lower range of the threshold suggested by Berg and Staff (1981), thus resulting in N loss under all the treatments. Although the C:N ratio (36.5) was higher than the threshold of 15, net N loss still occurred. A possible reason may be that N deposition positively affected the adaptation of the decomposer communities to low-N substrates and altered the stoichiometric requirement of the decomposers, thus facilitating the occurrence of N release. A similar result was also observed in our previous study (Song et al. 2014a). This result may imply that the microbial decomposers in Moso bamboo plantations have a higher stoichiometric threshold in decaying litter.
The effects of N addition on the activity of the soil microbial decomposers varied with the decay stage (Hobbie et al. 2012; Tu et al. 2012). In the early stage, N addition may increase the decomposers’ activity (Molina et al. 1983; Madritch and Hunter 2003) and promote N release. However, in the later stage, the cumulative N addition effect tends to induce C-based limitation of microbial decomposer activity (Thirukkumaran and Parkinson 2000; Andersson et al. 2004), limiting N release. In the present study, N loss in the CM plots was faster under the three N addition treatments than the control group in the first 8 months of decomposition but became significantly slower under N60 and N90 treatments after 12 months (Fig. 3). This pattern can be explained by early positive effects and the cumulative negative effects of N addition over time. In the IM plots, management practices may have improved microbial activity, and N addition, as another source of N, did not create positive effects on N loss in the early several months of decomposition but was associated with stronger negative effects in the later stage. The management practices may weaken the positive effects of N addition on N loss in the early stage of litter decomposition but enhance the negative effects in the later stage.
The C:P ratio regulates P release from decomposing litter. The critical C:P ratio, with a typical range between 200 and 480 (Gosz et al. 1973; Manzoni et al. 2010), varies across climatic regions and ecosystems. Tropical regions generally have a higher C:P ratio due to the compound effects of increased decomposer respiration, a high decomposer C:P ratio, and strong leaching (Manzoni et al. 2010). In the current study, the litter C:P ratio was far less than 480 and showed net P release from the beginning of the decomposition experiment under all treatments. The accumulation or release of P and N generally occur simultaneously (Manzoni et al. 2008, 2010). Unlike the effects on N loss in the later stage, management practices strengthened both the positive effects of N addition on P loss in the early stage and the negative effects in the later stage. In addition, physical processes, such as leaching, play an important role during decomposition in humid conditions, especially at the early stage of decomposition (Manzoni et al. 2010). In the present study, the leaching resulted from local abundant rainfall (Fig. 1) can also influence the effects of N addition on litter mass loss and nutrients release, which remains unclear.
Conclusions
Our P. pubescens leaf litter decomposition experiment over a 24-month period found that the low-N addition significantly accelerated leaf litter decomposition, whereas the high-N addition significantly retarded decomposition (P < 0.05). IM practices, such as weeding, ploughing, and fertilization, did not significantly influence decomposition alone but did amplify both the positive litter decomposition effects of the low-N addition and the negative litter decomposition effects of the high-N addition. N addition accelerated N and P loss in the early stage but suppressed N and P loss in the later stage. IM practices weakened the positive effects on N loss but enhanced the positive effects on P loss in the early stage and strengthened the negative effects on N and P loss in the later stage. The findings from this study provide a new perspective to better understand and estimate the biological effects of environmental change accompanying human management practices.
References
Andersson M, Kjøller A, Struwe S (2004) Microbial enzyme activities in leaf litter, humus and mineral soil layers of European forests. Soil Biol Biochem 36:1527–1537
Austin AT, Ballaré CL (2010) Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc Natl Acad Sci U S A 107:4618–4622
Balesdent J, Besnard E, Arrouays D, Chenu C (1998) The dynamics of carbon in particle-size fractions of soil in a forest-cultivation sequence. Plant Soil 201:49–57
Berg B, Staaf H (1981) Leaching, accumulation and release of nitrogen in decomposing forest litter. Ecol Bull 33:163–178
Brandt LA, King JY, Hobbie SE, Milchunas DG, Sinsabaugh RL (2010) The role of photodegradation in surface litter decomposition across a grassland Ecosystem precipitation gradient. Ecosystems 13:1–17
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Fang H, Mo J, Peng S, Li Z, Wang H (2007) Cumulative effects of nitrogen additions on litter decomposition in three tropical forests in southern china. Plant Soil 297:233–242
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli L, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Gosz JR, Likens GE, Bormann FH (1973) Nutrient release from decomposing leaf and branch litter in the Hubbard Brook Forest, New Hampshire. Ecol Monogr 43:173–191
Hobbie SE, Reich PB, Oleksyn J, Ogdahl M, Zytkowiak R, Hale C, Karolewski P (2006) Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87:2288–2297
Hobbie SE, Eddy WC, Buyarski CR, Adair EC, Ogdahl ML, Weisenhorn P (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr 82:389–405
Jiang P, Wang H, Wu J, Xu Q, Zhou G (2009) Winter mulch increases soil CO2 efflux under Phyllostachys praecox stands. J Soils Sediments 9:511–514
Keeler BL, Hobbie SE, Kellogg L (2009) Effects of long-term nitrogen additions on soil and litter microbial enzyme activity in eight forested and grassland sites–implications for litter and SOM decomposition. Ecosystems 12:1–15
Knorr M, Frey SD, Curtio PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Li H, Lei Y (2010) Estimation and evaluation of forest biomass carbon storage in China. Chinese Forestry Press, Beijing
Liu J, Jiang P, Wang H, Zhou G, Wu J, Yang F, Qian X (2011) Seasonal soil CO2 efflux dynamics after land use change from a natural forest to Moso bamboo plantations in subtropical China. For Ecol Manag 262:1131–1137
Liu X, Zhang Y, Han W, Tang A, Shen J, Cui Z, Vitousek P, Erisman JW, Goulding K, Christie P, Fangmeier A, Zhang F (2013) Enhanced nitrogen deposition over China. Nature 494:459–462
Lü C, Tian H (2007) Spatial and temporal patterns of nitrogen deposition in China: synthesis of observational data. J Geophys Res 112:10–15
Madritch MD, Hunter MD (2003) Intraspecific litter diversity and nitrogen deposition affect nutrient dynamics and soil respiration. Oecologia 136:124–128
Manning P, Saunders M, Bardgett RD, Bonkowski M, Bradford MA, Ellis RJ, Kandeler E, Marhan S, Tscherko D (2008) Direct and indirect effects of nitrogen deposition on litter decomposition. Soil Biol Biochem 40:688–698
Manzoni S, Jackson RB, Trofymow JA, Porporato A (2008) The global stoichiometry of litter nitrogen mineralization. Science 321:684–686
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80:89–106
Micks P, Downs MR, Magill AH, Nadelhoffer JK, Aber JD (2004) Decomposition litter as a sink for 15 N-enriched additions to an oak forest and a red pine plantation. For Ecol Manag 196:71–87
Mo J, Brown S, Xue J, Fang Y, Li Z (2006) Response of litter decomposition to simulated N deposition in disturbed rehabilitated and mature forests in subtropical china. Plant Soil 282:135–151
Molina JAE, Clapp CE, Shaffer MJ, Chichester FW, Larson WE (1983) A model of nitrogen and carbon transformations in soil: description, calibration and behaviour. Soil Sci Soc Am J 47:85–91
Olson JS (1963) Energy storage and the balance of producers and decomposition in ecological system. Ecology 44:322–331
Pancotto VA, Sala OE, Cabello M, Lopez M, Robson TM, Ballare C, Caldwell MM, Scopel A (2003) Solar UV-B decreases decomposition in herbaceous plant litter in Tierra del Fuego, Argentina: potential role of an altered decomposer community. Glob Chang Biol 9:1465–1474
Pandey RR, Sharma G, Tripathi SK, Singh AK (2007) Litterfall, litter decomposition and nutrient dynamics in a subtropical natural oak forest and managed plantation in northeastern India. For Ecol Manag 240:96–104
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brand LA, Hart SC (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364
Reay DS, Dentener F, Smith P, Grace J, Feely RA (2008) Global nitrogen deposition and carbon sinks. Nat Geosci 1:430–437
Rowland AP, Roberts JD (1994) Lignin and cellulose fractionation in decomposition studies using acid-detergent fibre methods. Commun Soil Sci Plan 25:269–277
Song X, Zhou G, Jiang H, Yu S, Fu J, Li W, Wang W, Ma Z, Peng C (2011) Carbon sequestration by Chinese bamboo forests, and their ecological benefits: assessment of potential, problems, and future challenges. Environ Rev 19:418–428
Song X, Jiang H, Zhang Z, Zhou G, Zhang S, Peng C (2014a) Interactive effects of elevated UV-B radiation and N deposition on Moso bamboo litter decomposition. Soil Biol Biochem 69:11–16
Song X, Zhang H, Jiang H, Peng C (2014b) Combination of nitrogen deposition and ultraviolet-B radiation decreased litter decomposition in subtropical China. Plant Soil 380:349–359
Thirukkumaran CM, Parkinson D (2000) Microbial respiration, biomass, metabolic quotient and litter decomposition in a lodgepole pine forest floor amended with nitrogen and phosphorous fertilizers. Soil Biol Biochem 32:59–66
Tu L, Hu H, Hu T, Zhang J, Xiao Y, Luo S, Li R, Dai H (2012) Effects of simulated nitrogen deposition on soil enzyme activities in a Betula luminifera plantation in Rainy Area of West China. Chin J Appl Ecol 23:2129–2134
Xie Y, Zhang S, Zhao X, Xiong Z, Xing G (2008) Seasonal variation patterns of NH4 +-N / NO3 −-N ratio and δ15 NH4 + value in rainwater in Yangtze river delta. Chin J Appl Ecol 19:2035–2041
Xu X, Hirata E (2005) Decomposition patterns of leaf litter of seven common canopy species in a subtropical forest: N and P dynamics. Plant Soil 273:279–289
Xu Q, Liu L, Hong Y (1998) Analysis on enzyme activities of high-yield and low-yield Phyllostachys pubescens stands. J Bamboo Res 17:37–40
Xu Q, Jiang P, Xu Z (2008) Soil microbial functional diversity under intensively managed bamboo plantations in southern China. J Soils Sediments 8:177–183
Xuan D, Song S, Yan Y, Weng J, Song X (2014) The short-term responses of soil enzyme activities in Moso bamboo forest to simulated nitrogen deposition. Ecol Sci 33:1122–1128
Xuluc-Tolosa FJ, Vester HFM, Ramirez-Marcial N, Castellanos-Albores J, Lawrence D (2003) Leaf litter decomposition of tree species in three successional phases of tropical dry secondary forest in Campeche, Mexico. For Ecol Manag 174:401–412
Zhou G, Xu J, Jiang P (2006) Effect of management practices on seasonal dynamics of organic carbon in soils under bamboo plantations. Pedosphere 16:525–531
Zhou G, Jiang P, Mo L (2009) Bamboo: a possible approach to the control of global warming. Int J Nonlinear Sci Numer Simul 10:547–550
Acknowledgments
This study was funded by the National Natural Science Foundation of China (Grant No. 31270517, 31470529), the “948” Project of the State Forestry Bureau of China (Grant No. 2013-4-55), and the Pandeng Project for Young & Middle-aged Discipline Leaders of Zhejiang Province (Grant No. pd2013234).
Compliance with Ethical Standards
The authors declare no competing financial interests. All of the authors have contributed substantially to the manuscript and approved the final submission. All previous work cited in the manuscript has been fully acknowledged.
This study did not involve human participants, specimens or tissue samples, or vertebrate animals, embryos or tissues.
This manuscript has not been submitted elsewhere in whole or in part and is not currently under consideration for publication in any other journal before a decision has been made by Plant and Soil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Per Ambus .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Song, X., Zhou, G., Gu, H. et al. Management practices amplify the effects of N deposition on leaf litter decomposition of the Moso bamboo forest. Plant Soil 395, 391–400 (2015). https://doi.org/10.1007/s11104-015-2578-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2578-2