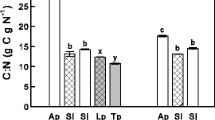
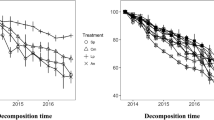
Abstract
Background
Litter decomposition is a fundamental process of biogeochemical cycles, and there is a strong consensus that litter mixture interactions are one of the factors driving the decomposition process. A better understanding of how climate change can alter interactions between species and the litter decomposition process could facilitate projections of ecosystem functioning into the future.
Methods
A 24-month litterbag decomposition experiment was carried out in a Mediterranean forest to analyze the effects of climate and species diversity changes on litter mixture interactions and the decomposition process.
Results
In the control plot, synergistic interactions increased with time and species diversity in litter mixtures, leading to more efficient litter decomposition. Drier conditions obtained in the field with a rain exclusion device decreased decomposition rates, resulting in three-fold less synergistic interactions and five-fold more antagonistic interactions during the decomposition process. Furthermore, synergistic interactions were better preserved in the drought conditions with increasing number of species.
Conclusions
Our findings underline how a longer drought season could strongly affect the relationship between biodiversity and ecosystem functioning. Drier climate led to slower mass loss rates and a strong shift in the litter mixture interactions, with fewer synergistic interactions and more antagonistic interactions.






Similar content being viewed by others
Abbreviations
- CV:
-
Coefficient of variation
- DSH:
-
Diversity-stability hypothesis
- IMC:
-
Improved microenvironmental condition
- NAE:
-
Non-additive effect
- RME:
-
Relative mixture effect
References
Adams RP (2007) Identification of essential oil components by gas chromatography / mass spectrometry, 4th edn. Allured Publishing Corporation, Carol Stream
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Almagro M, Martinez-Mena M (2012) Exploring short-term leaf-litter decomposition dynamics in a Mediterranean ecosystem: dependence on litter type and site conditions. Plant Soil 358:323–335
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442:555–558
Baize D, Girard MC (1998) A sound reference base for soils: the « référentiel pédologique ». INRA eds, Paris
Bardgett R (2005) The biology of soil: a community and ecosystem approach. Oxford University Press, Oxford
Bonanomi G, Incerti G, Antignani V, Capodilupo M, Mazzoleni S (2010) Decomposition and nutrient dynamics in mixed litter of Mediterranean species. Plant Soil 331:481–496
Cebrian J (1999) Patterns in the fate of production in plant communities. Am Nat 154:449–468
Chen BM, Peng SL, D’Antonio CM, Li DJ, Ren WT (2013) Non-additive effects on decomposition from mixing litter of the invasive Mikania micrantha H.B.K. with native plants. PLos One 8(6):e66289
Chomel M, Fernandez C, Bousquet-Melou A, Gers C, Monnier Y, Santonja M, Gauquelin T, Gros R, Lecareux C, Baldy V (2014) Secondary metabolites of Pinus halepensis alter decomposer organisms and litter decomposition during afforestation of abandoned agricultural zones. J Ecol 102:411–424
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, Van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Diaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaeieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Coulis M, Hättenschwiler S, Fromin N, David JF (2013) Macroarthropod-microorganism interactions during the decomposition of Mediterranean shrub litter at different moisture levels. Soil Biol Biochem 64:114–121
Couteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66
De Marco A, Meola A, Maisto G, Giordano M, De Santo AV (2011) Non-additive effects of litter mixtures on decomposition of leaf litters in a Mediterranean maquis. Plant Soil 344:305–317
Dirks I, Navon Y, Kanas D, Dumbur R, Grünzweig JM (2010) Atmospheric water vapor as driver of litter decomposition in Mediterranean shrublands and grassland during rainless seasons. Glob Chang Biol 16:2799–2812
Emberger C, Gaussen H, Kassas M, de Philippis A (1963) Bioclimatic map of the Mediterranean Zone, explanatory notes. UNESCO-FAO, Paris
Folin O, Denis W (1915) A colorimetric method for the determination of phenols (and phenol derivates) in urine. J Biol Chem 22:305–308
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–246
Gessner MO, Swan CM, Dang CK, Mckie BG, Bardgett RD, Wall DH, Hättenschwiler S (2010) Diversity meets decomposition. Trends Ecol Evol 25:372–380
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Chang Biol 6:751–765
Giorgi F, Lionello P (2008) Climate change projections for the Mediterranean region. Glob Planet Chang 63:90–104
Gobat JM, Aragno M, Matthey W (2010) Le sol vivant : bases de pédologie, biologie des sols, 3rd edn. Presse polytechniques et universitaires romandes, Lausanne
Harguindeguy NP, Blundo CM, Gurvich DE, Diaz S, Cuevas E (2008) More than the sum of its parts? Assessing litter heterogeneity effects on the decomposition of litter mixtures through litter chemistry. Plant Soil 303:151–159
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol Syst 36:191–218
Hättenschwiler S, Aeschlimann B, Couteaux MM, Roy J, Bonal D (2008) High variation in foliage and leaf litter chemistry among 45 tree species of a neotropical rainforest community. New Phytol 179:165–175
Hättenschwiler S, Coq S, Barantal S, Handa IT (2011) Leaf traits and decomposition in tropical rainforest: revisiting some commonly held views and towards a new hypothesis. New Phytol 189:950–965
Hector A, Beale AJ, Minns A, Otway SJ, Lawton JH (2000) Consequences of the reduction of plant diversity for litter decomposition: effects through litter quality and microenvironment. Oikos 90:357–371
Hilaire C, Orts JP, Boer B, Gauquelin T (2012) Le domaine de l’Observatoire de Haute-Provence (OHP): Hermas et chênaie pubescente, du XVIIIe siècle à nos jours. Courrier Scientifique du Parc Naturel Régional du Luberon et de la Réserve Biosphère Luberon-Lure 11:8–21
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setala H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Hoorens B, Aerts R, Stroetenga M (2002) Litter quality and interactive effects in litter mixtures: more negative interactions under elevated CO2? J Ecol 90:1009–1016
Hoorens B, Stroetenga M, Aerts R (2010) Litter mixture interactions at the level of plant functional types are additive. Ecosystems 13:90–98
Incerti G, Bonanomi G, Giannino F, Rutigliano FA, Piermatteo D, Castaldi S, De Marco A, Fierro A, Fioretto A, Maggi O, Papa S, Persiani AM, Feoli E, Virzo de Santo A, Mazzoleni S (2011) Litter decomposition in Mediterranean ecosystems: modeling the controlling role of climatic conditions and litter quality. Appl Soil Ecol 49:148–157
IPCC (Intergovernmental Panel on Climate Change) (2013) Climate change 2013—the physical science basis. Cambridge University Press, Cambridge
IUSS Working Group WRB (2006) World reference base for soil resources 2006 - world soil resources reports No. 103. FAO, Rome
Keith AM, van der Wal R, Brooker RW, Osler GHR, Chapman SJ, Burslem DFRP, Elston DA (2008) Increasing litter species richness reduces variability in a terrestrial decomposer system. Ecology 89:2657–2664
Knops JMH, Wedin D, Tilman D (2001) Biodiversity and decomposition in experimental grassland ecosystems. Oecologia 126:429–433
Kominoski JS, Pringle CM, Ball BA, Bradford MA, Coleman DC, Hall DB, Hunter MD (2007) Non-additive effects of leaf litter species diversity on breakdown dynamics in a detritus-based stream. Ecology 88:1167–1176
Lecerf A, Risnoveanu G, Popescu C, Gessner MO, Chauvet E (2007) Decomposition of diverse litter mixtures in streams. Ecology 88:219–227
Lensing JR, Wise DH (2007) Impact of changes in rainfall amounts predicted by climate-change models on decomposition in a deciduous forest. Appl Soil Ecol 35:523–534
Leroy CJ, Marks JC (2006) Litter quality, stream characteristics and litter diversity influence decomposition rates and macroinvertebrates. Freshw Biol 51:605–617
Maisto G, De Marco A, Meola A, Sessa L, De Santo AV (2011) Nutrient dynamics in litter mixtures of four Mediterranean maquis species decomposing in situ. Soil Biol Biochem 43:520–530
Makkonen M, Berg MP, van Logtestijn RSP, van Hal JR, Aerts R (2013) Do physical plant litter traits explain non-additivity in litter mixtures? A test of the improved microenvironmental conditions theory. Oikos 122:987–997
Mayfield MM, Bonser SP, Morgan JW, Aubin I, McNamara S, Vesk PA (2010) What does species richness tell us about functional trait diversity? Predictions and evidence for responses of species and functional trait diversity to land-use change. Glob Ecol Biogeogr 19:423–431
Melillo JM, Aber JD, Linkins AE, Ricca A, Fry B, Nadelhoffer KJ (1989) Carbon and nitrogen dynamics along the decay continuum: plant litter to soil organic matter. Plant Soil 115:189–198
Penuelas J, Estiarte M, Kimball BA, Idso SB, Pinter PJ, Wall GW, Garcia RL, Hansaker DJ, LaMorte RL, Hendrix DL (1996) Variety of responses of plant phenolic concentration to CO2 enrichment. J Exp Bot 47:1463–1467
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walter BH, Walker M, Wall DH (2000) Biodiversity—global biodiversity for the year 2100. Science 287:1770–1774
Sanpera-Calbet I, Lecerf A, Chauvet E (2009) Leaf diversity influences in-stream litter decomposition through effects on shredders. Freshw Biol 54:1671–1682
Sardans J, Penuelas J (2013) Pant-soil interactions in Mediterranean forest and shrublands: impact of climate change. Plant Soil 365:1–33
Saura-Mas S, Estiarte M, Penuelas J, Lloret F (2012) Effects of climate change on leaf litter decomposition across post-fire plant regenerative groups. Environ Exp Bot 77:274–282
Schimel JP, Hättenschwiler S (2007) Nitrogen transfer between decomposing leaves of different N status. Soil Biol Biochem 39:1428–1436
Schindler MH, Gessner MO (2009) Functional leaf traits and biodiversity effects on litter decomposition in a stream. Ecology 90:1641–1649
Schroter D, Cramer W, Leemans R, Prentice IC, Araujo MB, Arnell NW, Bondeau A, Bugmann H, Carter TR, Gracia CA, de la Vega-Leinert AC, Erhard M, Ewert F, Glendining M, House JI, Kankaanpaa S, Klein RJT, Lavorel S, Lindner M, Metzger MJ, Meyer J, Mitchell TD, Reginster I, Rounsevell M, Sabate S, Sitch S, Smith B, Smith J, Smith P, Sykes MT, Thonicke K, Thuiller W, Tuck G, Zaehle S, Zierl B (2005) Ecosystem service supply and vulnerability to global change in Europe. Science 310:1333–1337
Srivastava DS, Cardinale BJ, Downing AL, Duffy JE, Jouseau C, Sankaran M, Wright JP (2009) Diversity has stronger top-down than bottom-up effects on decomposition. Ecology 90:1073–1083
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. University of California Press, Berkeley
Throop HL, Archer SR (2007) Interrelationships among shrub encroachment, land management, and litter decomposition in a semidesert grassland. Ecol Appl 17:1809–1823
Tilman D, Reich PB, Knops JMH (2006) Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441:629–632
Tiunov AV, Scheu S (2005) Facilitative interactions rather than resource partitioning drive diversity‐functioning relationships in laboratory fungal communities. Ecol Lett 8:618–625
Van Soest PJ, Wine RH (1968) The determination of lignin and cellulose in acid detergent fiber with permanganate. J Assist Off Chem 51:780–787
Vogel A, Eisenhauer N, Weigelt A, Scherer-Lorenzen M (2013) Plant diversity does not buffer drought effects on early-stage litter mass loss rates and microbial properties. Glob Chang Biol 19:2795–2803
Vos VCA, van Ruijven J, Berg MP, Peeters ETHM, Berendse F (2013) Leaf litter quality drives litter mixing effects through complementary resource use among detritivores. Oecologia 173:269–280
Walter J, Hein R, Beierkuhnlein C, Hammerl V, Jentsch A, Schadler M, Schuerings J, Kreyling J (2013) Combined effects of multifactor climate change and land-use on decomposition in temperate grassland. Soil Biol Biochem 60:10–18
Wardle DA, Bonner KI, Nicholson KS (1997) Biodiversity and plant litter: experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos 79:247–258
Weltzin JF, Loik ME, Schwinning S, Williams DG, Fay PA, Haddad BM, Harte J, Huxman TE, Knapp AK, Lin GH, Pockman WT, Shaw MR, Small EE, Smith MD, Smith SD, Tissue DT, Zak JC (2003) Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience 53:941–952
Wieder WR, Cleveland CC, Townsend AR (2009) Control over leaf litter decomposition in wet tropical forests. Ecology 90:3333–3341
Wu DD, Li TT, Wan SQ (2013) Time and litter species composition affect litter-mixing effects on decomposition rates. Plant Soil 371:355–366
Acknowledgments
We thank Sylvie Dupouyet, Jean-Philippe Orts and Ilja Reiter for their contributions to the set-up of the experiment and the field work, and Caroline Lecareux and Germain Boungou for their assistance on the chemical analyses. We also thank Stephan Hättenschwiler for his contribution to the chemical analyses. This study was funded by the EC2CO-BIOEFFECT program (CNRS) and the French National Research Agency (ANR) through the SecPriMe2 project (ANR-12-BSV7-0016-01), the research federation ECCOREV FR3098 and the LABEX OT-Med. We also thank Europe and the PACA region of France for sponsoring Mathieu Santonja’s PhD work. Finally, we thank ATT (scientific language editing services) for proofreading the draft manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Rights and permissions
About this article
Cite this article
Santonja, M., Fernandez, C., Gauquelin, T. et al. Climate change effects on litter decomposition: intensive drought leads to a strong decrease of litter mixture interactions. Plant Soil 393, 69–82 (2015). https://doi.org/10.1007/s11104-015-2471-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2471-z