Abstract
Background and aims
Adequate zinc (Zn) in maize (Zea mays L.) is required for obtaining Zn-enriched grain and optimum yield. This study investigated the impact of varying Zn fertilizer placements on Zn accumulation in maize plant.
Methods
Two pot experiments with same design were conducted to investigate the effect of soil Zn heterogeneity by mixing ZnSO4·7H2O (10 mg Zn kg−1 soil on an average) in 10–15, 0–15, 25–30, 0–30, 30–60 and 0–60 cm soil layers on maize root growth and shoot Zn content at flowering stage in experiment-1, and assessing effects on grain Zn accumulation at mature stage in experiment-2.
Results
In experiment-1, Zn placements created a large variation in soil DTPA-Zn concentration (0.3–29.0 mg kg−1), which induced a systemic and positive response of root growth within soil layers of 0–30 cm; and shoot Zn content was increased by 102 %–305 % depending on Zn placements. Supply capacity of Zn in soil, defined as sum of product of soil DTPA-Zn concentration and root surface area at different soil layers, was most related to shoot Zn content (r = 0.82, P < 0.001) via direct and indirect effects according to path analysis. In experiment-2, Zn placements increased grain Zn concentration by up to 51 %, but significantly reduced the grain Zn harvest index from 50 % by control to about 30 % in average.
Conclusion
Matching the distribution of soil applied Zn with root by Zn placement was helpful to maximize shoot Zn content and grain Zn concentration in maize.
Similar content being viewed by others
Introduction
Human are now facing two global challenges from agriculture: one is to ensure food security, conserve natural resources and protect environments, simultaneously (Tilman et al. 2002); furthermore, another hidden and ignored one is to increase grain micronutrient (for example zinc, Zn) concentration in main cereal crops to overcome widespread malnutrition especially in developing countries (Bouis and Welch 2010). Maize (Zea mays L.) is one of the leading cereal crops worldwide, and its total production is more than that of any other cereal grain (FAO 2011). And it is also an important cereal crop in the context of nutrition for humans, poultry and livestock (Nuss and Tanumihardjo 2010). Thus increasing Zn levels in maize grain could deliver more Zn to people whose diet relies directly or indirectly on maize-derived food.
Maize is one of the most susceptible cereal crops to Zn deficiency. Because high yielding maize varieties are selected grown, chemical fertilizers are of increased purity and cropping has become increasingly intensive, Zn deficiency in soil-crop system has become more prevalent in last decades (Fageria et al. 2002). Zinc applications are reported to increase maize grain yield around world (Harris et al. 2007; Hossain et al. 2008; Potarzycki and Grzebisz 2009; Singh and Banerjee 1986). In China, nearly half of Chinese farmlands were classified as critical Zn deficient soil (Liu 1996), especially in North China where most of maize crops were planted. As summarized by Zou et al. (2008), cases of increase in maize yield by Zn application were frequently reported, and amount of Zn fertilizer used for maize production ranks first among all crops in China.
Meanwhile, Zn application was also an effective strategy of biofortification, which has been well documented to increase grain Zn concentration in wheat and rice (Cakmak 2008; Hossain et al. 2008; Shivay et al. 2008), but information specific to maize is limited. Recent studies indicated that it’s possible to increase Zn concentration in maize grain by either soil Zn application or seed priming with Zn in South Asia (Harris et al. 2007; Hossain et al. 2008).
However, only a small part of applied Zn is taken up by crops resulting in low apparent recovery rate of applied Zn, ranging from less than 1 % to 5 % depending on fertilizer types and rates (Rico et al. 1996; Zhao et al. 2011). This inefficiency in Zn use was partly due to both quick fixation of Zn into crop unavailable forms and the limited mobility of Zn fertilizer when applied as ZnSO4 (Alloway 2009; Rengel et al. 1999). Increasing rates of Zn fertilizers may supply more available Zn to crops, although it may not economical (Zhao et al. 2011). Alternatively, granular Zn fertilizer needs to be well mixed in the soil to maximize root interception of added Zn granules for increasing Zn use efficiency (Mortvedt and Gilkes 1993). In addition, a root plasticity (a trait that can respond to selective pressure and may help plants forage for nutrients in heterogeneous soils) response to soil patches enriched in macronutrients such as phosphate or ammonium may increase acquisition of nutrients (Drew 1975). Unlike the hyper-accumulator such as Thlaspi caerulescens, little is known about maize root plasticity response to local Zn enrichment in soil (Haines 2002; Robinson 1994). Considering these factors together, a mismatch between maize root distribution and Zn availability in the soil profile may constrain the uptake of Zn by maize root if there was no response to Zn-enriched patch. In addition, experiments with wheat showed that Zn addition in infertile subsoil could greatly increase root growth, shoot Zn accumulation and also grain yield for Zn inefficient wheat genotype (Holloway et al. 2010). Thus, maximizing shoot Zn uptake by efficient Zn placements including subsoil Zn application to meet the requirement of Zn-enriched grain and high yield of maize deserves further research.
Overall, it’s still unclear how soil Zn heterogeneity affects root growth, shoot Zn content, apparent recovery efficiency of applied Zn and also grain Zn concentration in maize. The objectives of this research, therefore, were to explore the effects of soil Zn heterogeneity by vertically stratified Zn on the plasticity of root distribution, shoot Zn accumulation and allocation in maize grown in pot culture conditions.
Materials and methods
Experimental design
Pot experiments were conducted in 2010 and 2011 at the solarium in Quzhou experimental station, Hebei province, China (36°52′N, 115°02′E). The experimental soil was a loamy textured alluvial Aquic Cambisol. The soil had an initial pH of 7.3 (soil to water ratio of 1:2), DTPA-extractable Zn concentration of 0.4 mg kg−1, Olsen-P concentration of 6.9 mg kg−1, total N concentration of 0.62 g kg−1, CaCO3 of 64.4 g kg−1and soil organic matter of 1.03 %. The soil was sieved through a 3 mm plastic sieve and air-dried prior to use. Pot experiments were carried out in cylindrical polyvinyl chloride (PVC) pots with 40-cm diameter and 75-cm depth (Fig. 1). Pots were divided into two parts along the diameter and then marked at 0 (soil surface), 5 (seed depth), 10, 15, 25, 30, 60, and 75 cm on the inside. After washing with diluted acid and then tap water, the pots were air dried and glued back by tape. The bottoms of pots were also airproofed by tape. The experiments consisted of seven vertical Zn placements with three replications: no Zn application (Zn0, as control), soil Zn application at 10–15 cm depth (Zn10–15, simulating banding application in topsoil), soil Zn application at 0–15 cm depth (Zn0–15, approximately simulating the broadcasting and mixing in topsoil), soil Zn application at 25–30 cm depth (Zn25–30, simulating deep banding in the plough layer), soil Zn application at 0–30 cm depth (Zn0–30, simulating broadcasting and mixing in the plough layer), soil Zn application at 30–60 cm depth (Zn30–60, simulating a subsoil fertilization) and soil Zn application throughout the 0–60 cm depth (Zn0–60, as complete Zn control). Zinc application rate was based on applying 10 mg Zn kg−1 soil in 0–60 cm soil depth (90 kg soil), while only NPK and no Zn was applied to 60–75 cm soil layer in all treatments. Therefore, Zn was applied at 900 mg Zn pot−1 as ZnSO4 · 7H2O for all Zn applications. The basal rates of fertilization were 100 mg N kg−1 soil as urea, 52 mg Pkg−1 soil as KH2PO4, and 125 (66 + 59) mg K kg−1 soil as KH2PO4 and K2SO4, all of which were applied and mixed evenly with soil before Zn application; then Zn fertilizer was applied as a Zn solution prepared with ZnSO4 · 7H2O and mixed evenly into NPK-fertilized soil. The prepared soil was added to PVC pots according to each treatment. Water at equivalence of 20 % gravimetric water content was added to the pots. Seeds of maize (Zea mays L., cv. “Zhengdan958”) were surface sterilized with a solution of 5 % H2O2 for 30 min. After washing by deionized water, four seeds were sown in each pot at 5 cm depth. After emergence, the seedlings were thinned to one per pot. All of the pots were watered with maximum to 40 mm per 2 or 3 days depending on weather conditions. At harvest in both years, no water-logging at bottom parts of columns was observed.
Sampling and measurement
In 2010, maize plants were harvested at flowering stage, which focuses on soil Zn availability, root growth, and uptake of shoot Zn in response to Zn placements (experiment-1, referred to as Exp-1). The shoots were washed with deionized water and then oven-dried to measure dry weight (DW) and nutrient concentration. The soil in each pot was divided into different samples representing the soil layers including 0–10, 10–15, 15–25, 25–30, 30–60, and 60–75 cm in order to measure DTPA extractable Zn in soil. The roots in the soil layers were sorted by hand and washed to measure the root length (RL) and root surface area (RSA) using a scanner (Epson, Japan) and root measurement software (RHIZO 4b, Australia). After scanning, the roots were dried at 65–70 °C in an oven and weighed.
In 2011, maize plants were grown to physiological maturity, focusing on grain yield, Zn accumulation in grain and other parts (experiment-2, referred to as Exp-2, which was exactly the same as in Exp-1 except the harvest stage). The shoot was divided into straw and grain to separately measure the dry weight, Zn concentration and content. The sampling of soil was conducted using the same method described in Exp-1.
Zinc concentrations in straw and grain samples were analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES, Perkin Elmer, USA) after digestion with HNO3-H2O2 in microwave accelerated system (CEM, Matthews, USA) (website, http://cem.com/). The recommended CEM method was: holding 2 min at 120 °C, then holding 5 min at 150 °C, finally holding 20 min at 185 °C and later with a process of cool down. The filtrated soil extract with DTPA solution was also measured by ICP-AES. Reference material IPE 556 (Wageningen University, Netherlands) was used to verify the digestion procedures and to calibrate the ICP-AES.
Calculation
Zinc contents in straw and grain were calculated as the product of Zn concentration and its dry weight. Grain Zn harvest index (Zn HI) was calculated as grain Zn content/shoot Zn content × 100. According to method of Zhu et al. (2001), specific Zn uptake (SZnU) was calculated as shoot Zn uptake per unit root DW. Due to the importance of soil Zn availability (Lindsay and Norvell 1978) and root surface area (Genc et al. 2007) on Zn uptake by plants, the sum of the product of soil available Zn concentration and root surface area at different soil layers was defined to quantify supply capacity of Zn (SCZn) in soil profile. The formula of SCZn was shown as follows:
where DTPA-Zn is soil DTPA-Zn concentration and RSA is root surface area in soil layers, which includes the 0–10, 10–15, 15–25, 25–30, 30–60 and 60–75 cm layers. Apparent recovery efficiency of applied Zn fertilizer (REZn) was calculated as (shoot Zn content with Zn application–shoot Zn content without Zn application)/amount of applied Zn × 100. Increase of shoot Zn content, SCZn, soil DTPA-Zn content was calculated as the difference value (△) between the respective values with and without Zn application.
Data analysis
One-factor ANOVA procedure in SAS software (SAS 8.0, USA) was used for statistical analysis. Means were separated by Fisher’s protected least significance difference (LSD) test at P < 0.05. Regression models were used to evaluate the response using SigmaPlot (Systat software, USA). Correlation studies and decomposition of coefficient between shoot Zn content and root characteristics by path analysis (to describe the directed dependencies among a set of variables, Hashimoto et al. 2012) were carried out using SAS software.
Results
Overall effect of Zn placement on maize growth
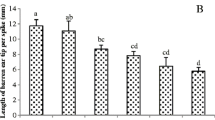
Symptoms of Zn deficiency were observed on maize leaves (e.g., interveinal chlorosis) at 4-leaf stage without Zn treatment (Fig. 1S, online data), whereas this symptom disappeared with time. Zn applications finally had no significant effect on total shoot dry weight (DW) in both experiments except Zn10–15 treatment in 2011, and on grain DW in Exp-2 comparing with control (Fig. 2; Table 1). There was a significant difference in root DW, root length and root surface area between control and Zn application treatments in Exp-1, whereas no or minor differences in these parameters occurred among Zn applications (Table 1). In addition, shoot/root ratio was significantly decreased by Zn applications except for Zn30–60 treatment (Table 1).
Effect of Zn placement on shoot (straw + grain) and grain dry weight at maize maturity in Exp-2. Columns with same letters are not significant at P < 0.05. Treatments are the same with Table 1
Heterogeneity of soil DTPA-Zn in treated soil layers
Soil DTPA-Zn concentrations in soil layers with applied Zn were significantly increased, thereby generating substantial Zn heterogeneity. Whereas DTPA-Zn in soil layers without Zn application had no difference compared with control treatment in two experiments (Fig. 3). The highest DTPA-Zn concentration was 28.3 mg kg−1 in 25–30 cm soil layer of Zn25–30 treatment at flowering stage in 2010, or 29.0 mg kg−1 in 10–15 cm soil layer of Zn10–15 treatment at maturity in 2011, which was nearly 70 times higher than that of control. Soil DTPA-Zn concentration increased linearly with increasing rates of soil Zn application resulting from the reduced soil volume of Zn mixing (Fig. 2S, online data).
Effect of Zn placement on soil DTPA-Zn concentration of different soil layers at maize flowering stage in 2010 (Exp-1) and at maturity in 2011 (Exp-2). Treatments are the same with Table 1
Root length density and root surface area in soil profile
For all treatments in Exp-1, the biggest root length density was in soil layer of 0–10 cm following by soil layer of 25–30 cm, and the lowest was in soil layer of 60–75 cm (Fig. 4). Compared with Zn0 treatment, all of Zn application treatments resulted in significantly higher root length density within soil layers of 0–30 cm, whereas Zn had no significant effect on root length density observed in30–75 cm soil layers. Overall, Zn application, regardless of its distribution in soil, resulted in a systemic not localized promotion of root growth (Table 1; Fig. 4). For example, Zn10–15 treatment resulted in similar root length density in soil layer of 10–15 cm as that with other Zn treatments, although soil DTPA-Zn concentration in soil layer of 10–15 cm was much higher than others (Fig. 3). For absolute quantity of root surface area (RSA) in soil profile, soil layers of 0–10 and 30–60 cm had much more RSA than other soil layers (Fig. 4).
Effect of Zn placement on root length density and root surface area in different soil layers at maize flowering stage in Exp-1. Treatments are the same with Table 1
Shoot Zn accumulation and allocation
There was a large variation in shoot Zn concentration and content among treatments in two experiments (Tables 2 and 3). Treatment Zn0–30 followed by Zn0–15 in Exp-1, and treatment Zn0–60 followed by Zn0–30 in Exp-2 resulted in highest shoot Zn concentration and content; while treatment Zn30–60 in both experiments had the lowest shoot Zn concentration and content. Similar to shoot Zn content, specific Zn uptake (SZnU) was significantly increased by Zn applications compared with that of control. A significant difference in SZnU among Zn application treatments was found, with the largest value in Zn0–30 treatment and lowest in Zn30–60 treatment (Table 2). Generally, a low apparent recovery efficiency of applied Zn (REZn) was recorded in all Zn treatments with ranges of 0.26–0.76 % in Exp-1 and 0.25–0.75 % in Exp-2 (Tables 2 and 3).
In Exp-2, grain Zn concentrations were obviously increased in all treatments except in the Zn30–60 treatment (Table 3). Treatment Zn0–15 followed by Zn0–30 resulted in the largest increase in grain Zn concentration, which was 51.2 % and 48.5 % higher than the control (Zn0), respectively. There was a significant and positive correlation between shoot Zn concentration and grain Zn concentration (P < 0.001), although grain Zn HI was significantly decreased by all Zn application treatments (Table 3).
Contribution of tested parameters to shoot Zn uptake
In Exp-1, Zn applications increased soil DTPA-Zn content to 7–12 folds higher than that of control. Difference in soil DTPA-Zn content among treatments with Zn application was small (Table 2). Zinc placement had a large effect on the supply capacity of Zn (SCZn) in soil layers where Zn was applied (Table 4). All Zn applications increased total SCZn up to 10 fold higher than Zn0 treatment.
A correlation matrix shown in Table 5 reveals that shoot Zn content was positively correlated with SCZn, weakly correlated with soil DTPA-Zn content and RSA, but not with RL. The coefficients were further estimated by path analysis (Table 6). SCZn had highest direct effect on shoot Zn content; soil DTPA-Zn content, RL and RSA contributed to shoot Zn content mostly by an indirect effect via SCZn. SCZn followed by soil DTPA-Zn content and RSA had a greater total effect on shoot Zn content than that of RL.
Increase in shoot Zn content, as an indicator of maximizing shoot Zn content, was negatively related to the increase in soil DTPA-Zn content, whereas it had a positive regression with SCZn in Exp-1(Fig. 5).
Discussion
In current study, Zn deficiency in soil obviously restricted the growth of maize seedling (Fig. 1S) and its root (Fig. 4; Table 1), and resulted in a critically low shoot Zn concentration (<15 mg kg−1, Broadley et al. 2007). However, low soil Zn ultimately had a minor effect on shoot or grain DW in two experiments (Fig. 2; Table 1). One reason may be that cultivar Zhengdan958 is one of most adopted cultivars in China, which is also a moderately Zn efficient genotype (Karim et al. 2012). In addition, sufficient soil moisture can reduce crop Zn deficiency by improving Zn diffusion in rhizosphere and thus increasing Zn uptake by root (Bagci et al. 2007). Thus, yield response to Zn application may well be different in field conditions. For example, an ongoing field experiment using the same cultivar and the same soil in Quzhou station indicated that maize grain yield could be increased by up to 12 % after heavy Zn application (50–150 kg ZnSO4 · 7H2O ha−1) (unpublished data).
The present study indicated that Zn application greatly increased soil DTPA-Zn concentration by Zn placement in columns (Fig. 3). Vertical heterogeneity of soil DTPA-Zn can be usually found between topsoil and subsoil because of surface cultivation and fertilization (Behera et al. 2008; Wei et al. 2006). This study provided a test of effects of a large amount of vertical stratification in soil DTPA-Zn concentration in soil layers, which reflects low mobility of inorganic Zn fertilizers in calcareous soils with high pH and high CaCO3 content (Alvarez 2007; Gangloff et al. 2006). Therefore localized placement of a constant amount of Zn fertilizer per pot resulted in a “concentration effect” (Fig. 2S), even with frequent irrigation.
Exp-1 was conducted to provide insight into the effects of Zn placement on maize roots. Root morphology as described by root DW, root length and root surface area was affected by Zn applications, but differences were minor among treatments with different Zn placements (Table 1). Increase in root length and root surface area by Zn application was previously reported (Subramanian et al. 2008; Taheri et al. 2011). A reason would be that Zn application increased the production and activity of indole-3-acetic acid, which promotes root growth (Alloway 2008). However, the large increase of soil DTPA-Zn in specific layers due to localized Zn application did not result in local root proliferation as indicated by root length density or root surface area (Fig. 4). Thus, the present finding further confirmed that positive effect of localized Zn application on root growth is not a localized but systemic response, which differed from local root proliferation associated with P placement (Drew 1975; Jing et al. 2010). The root plasticity related to local high nutrient concentration reviewed by Robinson (1994), did not seem to occur with localized Zn application to maize. But the root increase by Zn applications resulted in reduced shoot/root ratio which showed a significantly negative correlation with shoot Zn content (r = 0.49*, data not shown); Moreover, Zn applications increased root growth predominately in soil layers of 0–30 cm, which was more important for Zn uptake (Table 1S, online data). Such result highlights importance of Zn application at surface soil (within 0–30 cm depth), although the underlying mechanism is not well understood. In addition, root length density showed a distinct peak at 25–30 cm depth in all Zn treatments, but not in control (Fig. 4). These observations occurred at flowering stage when the tips of brace root may be just rooted in 25–30 cm depth. Another explanation would be that root system of modern maize cultivars is adapted to nutrient-enriched topsoil (e.g., 0–30 cm) during varietal selection (Dwyer et al. 1996; Peng et al. 2010).
Although the same amount of Zn sulfate was applied in Zn application treatments, variation in shoot Zn content of maize was huge in both experiments (Tables 2 and 3). The varied SZnU indicated that influx rate of Zn in root was significantly increased by Zn placement. Nonetheless, variation of shoot Zn content by Zn placement, low REZn (Tables 2 and 3), Zn influx characterized by Michaelis–Menten functions (Broadley et al. 2007) and maize being not a hyper-accumulation species of Zn (Takkar and Mann 1978) support the idea that ability of roots to take up Zn from local high Zn concentration is still a limitation for maximizing shoot Zn content. On the basis of poor mobility of inorganic Zn fertilizer in soil and the systemic response of maize root to Zn placement, we speculated that depth-wise matching between root and available Zn in soil contributed to this variation in shoot Zn content among Zn treatments. Treatments where Zn was applied throughout 0–60 cm depth or where it was applied only to 0–30 (or 0–15) cm resulted in higher shoot Zn content. Where Zn was applied only in 30–60 cm zone resulted in lowest Zn content in both experiments. Few studies can be comparable with current results because previous studies focused mostly on new types of fertilizer, correction of Zn deficiency, new methods such as seed priming (Alvarez and Rico 2003; Harris et al. 2007; Hossain et al. 2008), but not the vertical stratification of Zn fertilizer. Both root surface area (Genc et al. 2007) and available Zn in the soil (e.g., DTPA-Zn) (Lindsay and Norvell 1978) are essential for Zn uptake by plants. Across all treatments, root surface area and DTPA-Zn content were weakly correlated with shoot Zn content (Table 5). However, in terms of maximizing shoot Zn content, increase of DTPA-Zn content had a negative regression with shoot Zn content (Fig. 5), indicating that only increases in soil DTPA-Zn content without concordant distribution of root does not ensure higher shoot Zn content. The expression of supply capacity of Zn in soil (SCZn), therefore, was introduced to quantify the spatial matching between root surface area and soil DTPA-Zn concentration. Across all treatments in Exp-1, value of SCZn varied with Zn placement (Table 3), and was significantly correlated with shoot Zn content (Table 5) and contributed to shoot Zn content most via direct effect (Table 6). Furthermore, increase in SCZn was positively correlated with increases in shoot Zn content to some extent (Fig. 5), indicating that optimizing spatial matching between root and soil available Zn distribution could increase shoot Zn content of maize. However, it’s noticeable that SCZn could only explain about 66 % (r2 between SCZn and shoot Zn content in Table 5) of the variation in shoot Zn content, indicating that factors such as rate of Zn influx, period of Zn uptake, soil moisture and sulfate heterogeneity in soil profile may be also involved and could not been excluded in this study. Specially, notable differences in shoot Zn content between Zn10–15 (Zn25–30) and Zn0–15 (Zn0–30) treatments were observed in Exp-1 but not in Exp-2 (Tables 2 and 3). This may suggest that difference in climate and harvesting stage between the 2 years and growth of brace root affected the Zn uptake after flowering. In addition, only 8 % of total sulfate was derived from zinc sulfate, thus the effect of sulfate heterogeneity seems small. Overall, these results supported the idea that method of Zn fertilization, especially fertilizer placement with inorganic Zn fertilizer (commonly as ZnSO4) should be considered in order to maximize shoot Zn content to meet crop requirement. For example, subsoil Zn fertilization at 30–60 cm soil depth is not recommended based on shoot Zn uptake and also is not feasible in practice, which was different with subsoil Zn addition for wheat production (Holloway et al. 2010; Nable and Webb 1993).
In field conditions, maize roots are generally distributed throughout the topsoil layers. For example, nearly 90 % of the root system was recovered from topsoil layers (Dwyer et al. 1996) and nearly half of the maize roots were recovered from soil layers of 0–15 cm (Peng et al. 2010). Thus, Zn application evenly within soil layers of 0–30 cm or alternatively 0–15 cm (by spraying Zn solutions on the topsoil before plowing or by broadcasting Zn-enriched NPK fertilizer and then mixing it into plow layer during field preparation) is expected to match well with root distribution, which would be expected to best enable Zn uptake by roots and, therefore, accumulation into the shoot. Furthermore, low in-season REZn (<1 %) matched with previous studies (Zhao et al. 2011; Wang et al. 2012), indicating that considerable amount of available Zn was retained in soil and a residual effect of Zn application therefore would be expected.
Compared with control, grain Zn concentration was obviously increased by Zn placement with the exception of the treatment Zn30–60 in Exp-2 (Table 3). Recent studies also confirmed that Zn application can increase Zn concentration in maize grain either by soil application or seed priming with Zn (Harris et al. 2007; Hossain et al. 2008). This study together with those studies suggest that biofortification of maize with Zn by an agronomic approach is also promising as in the case of wheat (Cakmak 2008). Without Zn application, grain Zn HI was about 50 %, similar with previous studies (Jarausch-Wehrheim et al. 1999; Wang et al. 2012). However, a larger portion of Zn was allocated to vegetative organs with declining grain Zn HI when Zn was applied irrespective of placement (Table 3). This phenomenon was reported before in maize (Jarausch-Wehrheim et al. 1999), rice (Jiang et al. 2008), and wheat (Yilmaz et al. 1997). Since Zn concentrations in the shoots could be increased by Zn application, thus Zn homeostasis by Zn loading in phloem, translocation and unloading to grain would be one limitation (Herren and Feller 1997; Stomph et al. 2011), and the limited sink due to low concentration of N and P which can store Zn in grain would be another limitation (Cakmak 2008; Nuss and Tanumihardjo 2010). Overall, grain Zn concentration combining the current cultivar and Zn fertilization could not meet the target of maize biofortification with Zn, such as to 38 mg Zn kg−1 (Bouis and Welch 2010). In addition, it’s noticeable that Zn30–60 treatment had no effect on grain Zn concentration; although it significantly increased shoot Zn content (Table 3). The reason was still unclear, it may be due to stronger suppression of Zn translocation to grain or the Zn uptake after anthesis was not used for grain Zn accumulation? The physiological process underlying translocation or retranslocation of Zn to grain deserves more research for successful biofortification of maize grain with Zn for human health.
Conclusion and prospect
This study was designed to investigate how soil Zn heterogeneity by Zn stratification affects root growth, and consequently shoot Zn uptake of maize in pot experiments. Mixing of ZnSO4 · 7H2O in soil induced a systemic and positive response of root growth mainly within the 0–30 cm soil depth. Spatial matching between soil available Zn and root distribution, termed supply capacity of Zn in soil, largely decided the shoot Zn accumulation. Increase of shoot Zn content by Zn enrichment in soil generally resulted in higher grain Zn concentration (except Zn30–60 treatment).
With the aim of grain Zn biofortification and sustainable high grain yield, Zn placement with feasible application practices (e.g., broadcasting Zn-contained NPK fertilizer or spraying Zn solution on soil surface and then incorporated into 15 or 30 cm depths by plow) for maximizing shoot Zn uptake of maize deserves further research in field conditions.
References
Alloway BJ (2008) Zinc in soils and crop nutrition. 2nd ed. International Zinc Association, Brussels; International Fertilizer Industry Association, Paris
Alloway BJ (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health 31:537–548
Alvarez JM (2007) Influence of soil type on the mobility and bioavailability of chelated Zinc. J Agric Food Chem 55:3568–3576
Alvarez JM, Rico MI (2003) Effects of zinc complexes on the distribution of zinc in calcareous soil and zinc uptake by maize. J Agric Food Chem 51:5760–5767
Bagci SA, Ekiz H, Yilmaz A, Cakmak I (2007) Effects of zinc deficiency and drought on grain yield of field-grown wheat cultivars in Central Anatolia. J Agron Crop Sci 193:198–206
Behera SK, Singh D, Dwivedi BS, Singh S, Kumar K, Rana DS (2008) Distribution of fractions of zinc and their contribution towards availability and plant uptake of zinc under long-term maize (Zea mays L.)-wheat (Triticum aestivum L.) cropping on an Inceptisol. Aust J Soil Res 46:83–89
Bouis HE, Welch RM (2010) Biofortification—a sustainable agricultural strategy for reducing micronutrient malnutrition in the Global South. Crop Sci 50:S20–S32
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302:1–17
Drew MC (1975) Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol 75:479–490
Dwyer LM, Ma BL, Stewart DW, Hayhoe HN, Balchin D, Culley JLB, McGovern M (1996) Root mass distribution under conventional and conservation tillage. Can J Soil Sci 76:23–28
Fageria NK, Baligar C, Clark RB (2002) Micronutrients in crop production. Adv Agron 77:185–268
FAO (2011) FAO Statistical Year. http://faostat.fao.org
Gangloff WJ, Westfall DG, Peterson GA, Mortvedt JJ (2006) Mobility of organic and inorganic zinc fertilizers in soils. Commun Soil Sci Plant Anal 37:199–209
Genc Y, Huang CY, Langridge P (2007) A study of the role of root morphological traits in growth of barley in zinc-deficient soil. J Exp Bot 58:2775–2784
Haines BJ (2002) Zincophilic root foraging in Thlaspi caerulescens. New Phytol 155:363–372
Harris D, Rashid A, Mira G, Arif M, Shah H (2007) ‘On-farm’ seed priming with zinc sulphate solution—a cost-effective way to increase the maize yields of resource-poor farmers. Field Crops Res 102:119–127
Hashimoto Y, Kang J, Matsuyama N, Saigusa M (2012) Path analysis of phosphorus retention capacity in allophanic and non-allophanic andisols. Soil Sci Soc Am J 76:441–448
Herren T, Feller U (1997) Influence of increased Zinc levels on phloem transport in wheat shoots. J Plant Physiol 150:228–231
Holloway RE, Graham RD, McBeath TM, Brace DM (2010) The use of a zinc-efficient wheat cultivar as an adaptation to calcareous subsoil: a glasshouse study. Plant Soil 336:15–24
Hossain MA, Jahiruddin M, Islam MR, Mian MH (2008) The requirement of zinc for improvement of crop yield and mineral nutrition in the maize-mungbean-rice system. Plant Soil 306:13–22
Jarausch-Wehrheim B, Mocquot B, Mench M (1999) Absorption and translocation of sludge-borne zinc in field-grown maize (Zea mays L.). Eur J Agron 11:23–33
Jiang W, Struik PC, van Keulen H, Zhao M, Jin LN, Stomph TJ (2008) Does increased zinc uptake enhance grain zinc mass concentration in rice? Ann Appl Biol 153:135–147
Jing JY, Rui YK, Zhang FS, Rengel Z, Shen JB (2010) Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Res 119:355–364
Karim MR, Zhang YQ, Tian D, Chen FJ, Zhang FS, Zou CQ (2012) Genotypic differences in zinc efficiency of Chinese maize evaluated in a pot experiment. J Sci Food Agr 92:2552–2559
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Liu Z (1996) Microelements in soils of China. Jiangsu Science and Technology Publishing House, Nanjing, China
Mortvedt JJ, Gilkes RJ (1993) Zinc fertilizers. In: Robson AD (ed) Zinc in soils and plants. Kluwer, The Netherlands, pp 33–44
Nable R, Webb M (1993) Further evidence that zinc is required throughout the root zone for optimal plant growth and development. Plant Soil 150:247–253
Nuss ET, Tanumihardjo SA (2010) Maize: a paramount staple crop in the context of global nutrition. Comp Rev Food Sci Food Saf 9:417–436
Peng YF, Niu JF, Peng ZP, Zhang FS, Li CJ (2010) Shoot growth potential drives N uptake in maize plants and correlates with root growth in the soil. Field Crops Res 115:85–93
Potarzycki J, Grzebisz W (2009) Effect of zinc foliar application on grain yield of maize and its yielding components. Plant Soil Environ 55:519–527
Rengel Z, Batten GD, Crowley DE (1999) Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crops Res 60:27–40
Rico MI, Alvarez JM, Mingot JI (1996) Efficiency of zinc ethylenediaminetetraacetate and zinc lignosulfonate soluble and coated fertilizers for maize in calcareous soil. J Agric Food Chem 44:3219–3223
Robinson D (1994) The response of plants to non-uniform supplies of nutrinet. New Phytol 127:635–674
Shivay YS, Kumar D, Prasad R, Ahlawat IPS (2008) Relative yield and zinc uptake by rice from zinc sulphate and zinc oxide coatings onto urea. Nutr Cycl Agroecosyst 80:181–188
Singh K, Banerjee NK (1986) Growth and zinc content of maize (Zea mays L.) as related to soil-applied zinc. Field Crops Res 13:55–61
Stomph TJ, Choi EY, Stangoulis JCR (2011) Temporal dynamics in wheat grain zinc distribution: is sink limitation the key? Ann Bot 107:927–937
Subramanian K, Bharathi C, Jegan A (2008) Response of maize to mycorrhizal colonization at varying levels of zinc and phosphorus. Biol Fertil Soils 45:133–144
Taheri S, Khoshgoftarmanesh A, Shariatmadari H, Chaney R (2011) Kinetics of zinc release from ground tire rubber and rubber ash in a calcareous soil as alternatives to Zn fertilizers. Plant Soil 341:89–97
Takkar PN, Mann MS (1978) Toxic levels of soil and plant zinc for maize and wheat. Plant Soil 49:667–669
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418:671–677
Wang JW, Mao H, Zhao HB, Huang DL, Wang ZH (2012) Different increases in maize and wheat grain zinc concentrations caused by soil and foliar applications of zinc in Loess Plateau, China. Field Crop Res 135:89–96
Wei XR, Hao MD, Shao MG, Gale WJ (2006) Changes in soil properties and the availability of soil micronutrients after 18 years of cropping and fertilization. Soil Tillage Res 91:120–130
Yilmaz A, Ekiz H, Torun B, Gultekin I, Karanlik S, Bagci SA, Cakmak I (1997) Effect of different zinc application methods on grain yield and zinc concentration in wheat cultivars grown on zinc-deficient calcareous soils. J Plant Nutr 20:461–471
Zhao AQ, Lu XC, Chen ZH, Tian XH, Yang XW (2011) Zinc fertilization methods on zinc absorption and translocation in wheat. J Agric Sci 3:28–35
Zhu YG, Smith SE, Smith FA (2001) Zinc (Zn)-phosphorus (P) interactions in two cultivars of spring wheat (Triticum aestivum L.) differing in P uptake efficiency. Ann Bot 88:941–945
Zou CQ, Gao XP, Shi RL, Fan XY, Zhang FS (2008) Micronutrient deficiencies in crop production in China. In: Alloway BJ (ed) Micronutrient deficiencies in global crop production. Springer, Netherlands, pp 127–148
Acknowledgements
This research was supported by the 973 project (No. 2009CB118605), China Agriculture Research System (CARS-02), the National Natural Science Foundation of China (31272252), and the Innovative Group Grant of NSFC (31121062).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ismail Cakmak.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1485 kb)
Rights and permissions
About this article
Cite this article
Zhang, YQ., Pang, LL., Yan, P. et al. Zinc fertilizer placement affects zinc content in maize plant. Plant Soil 372, 81–92 (2013). https://doi.org/10.1007/s11104-013-1904-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1904-9