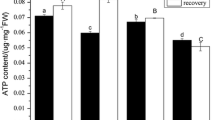
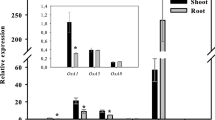
Abstract
The plasma membrane (PM) H+ ATPase is involved in the plant response to nutrient deficiency. However, adaptation of this enzyme in monocotyledon plants to phosphorus (P) deficiency lacks direct evidence. In this study, we detected that P deficient roots of rice (Oryza Sativa L.) could acidify the rhizosphere. We further isolated the PM from rice roots and analyzed the activity of PM H+ ATPase. In vitro, P deficient rice roots showed about 30% higher activity of PM H+ ATPase than the P sufficient roots at assay of pH 6.0. The P deficiency resulted in a decrease of the substrate affinity value (K m ) of PM H+ ATPase. The proton pumping activity of membrane vesicles from the P deficient roots was about 70% higher than that from P sufficient roots. Western blotting analysis indicated that higher activity of PM H+ ATPase in P deficient roots was related to a slightly increase of PM H+ ATPase protein abundance in comparison with that in P sufficient roots. Taken together, our results demonstrate that the P deficiency enhanced activities of both PM H+-ATPase and H+ pump, which contributed to the rhizosphere acidification in rice roots.




Similar content being viewed by others
References
Ai P, Sun S, Zhao J, Fan X, Xin W, Guo Q, Yu L, Shen Q, Wu P, Miller AJ, Xu GH (2009) Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J 57:798–809
Baginski ES, Foa PP, Zak B (1967) Determination of phosphate: study of labile organic phosphate interference. Clin Chim Acta 15:155–158
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constrainsts: a review. Plant Soil 248:43–59
Johansson F, Sommarin M, Larsson C (1993) Fusicoccin activates the plasma membrane H+-ATPase by a mechanism involving the C-terminal inhibitory domain. Plant Cell 5:321–327
Johansson F, Olbe M, Sommarin M, Larsson C (1995) Brij 58, a polyoxyethylene acyl ether, creates membrane vesicles of uniform sidedness. A new tool to obtain inside-out (cytoplasmic side-out) plasma membrane vesicles. Plant J 7:165–173
Kirk GJK, Saleque MA (1995) Solubilization of phosphate by rice plants growing in reduced soil: prediction of the amount solubilized and the resultant increased in uptake. Eur J Soil Sci 46:247–255
Kirk GJK, Santos EE, Findenegg GR (1999a) Phosphate solubilization by organic anion excretion from rice (Oryza sativa L.) growing in aerobic soil. Plant Soil 211:1–18
Kirk GJK, Santos EE, Satos MB (1999b) Phosphate solubilization by organic anion excretion from rice roots in aerobic soil: rates of excretion and decomposition effects on rhizosphere pH, and effects on phosphate solubility and uptake. New Phytol 128:469–477
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Larsson C (1985) Plasma membrane. In: Linskens HF, Jackson JF (eds) Modern methods of plant analysis. Springer, Berlin, pp 85–104
Li BZ, Wang SW, Feng HM, Xu GH (2008) Effects of nitrogen forms on root morphology and phosphate uptake in rice. Chin J Rice Sci 22:665–668
Liao H, Wan H, Shaff J, Wang X, Yan X, Kochian LV (2006) Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system. Plant Physiol 141:674–684
Marschner H (1995) In: Marschener H (ed) Mineral nutrition of higher plants, 2nd edn. Academic Press, London, pp 231–254
Mengel K, Schubert S (1985) Active extrusion of protons into deionized water by roots of intact maize plants. Plant Physiol 79:344–348
Michelet B, Boutry M (1995) The plasma membrane H+-ATPase: a highly regulated enzyme with multiple physiological functions. Plant Physiol 108:1–6
Moorby H, Nye P, White R (1985) The influence of nitrate nutrition on H+ efflux by young rape plants (Brassica napus cv. emerald). Plant Soil 84:403–415
Neumann G, Römheld V (1999) Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 211:121–130
Oufattole M, Arango M, Boutry M (2000) Identification and expression of three new Nicotiana plumbaginifolia genes which encode isoforms of a plasma-membrane H+-ATPase, and one of which is induced by mechanical stress. Planta 210:715–722
Palmgren MG (1998) Proton gradient and plant growth: role of the plasma membrane H+-ATPase. Adv Bot Res 28:1–70
Palmgren MG (2001) Plant plasma membrane H+-ATPase: powerhouses for nutrient uptake. Annu Rev Plant Mol Biol 52:817–845
Palmgren MG, Christensen G (1994) Functional comparison between plant plasma membrane H+-ATPase isoforms expressed in yeast. J Biol Chem 269:3027–3033
Palmgren MG, Sommarin M (1989) Lysophosphatidylcholine stimulates ATP dependent proton accumulation in isolated oat root plasma membrane vesicles. Plant Physiol 90:1009–1014
Palmgren MG, Sommarin M, Serrano R, Larsson C (1991) Identification of an autoinhibitory domain in the C-terminal region of the plant plasma membrane H+-ATPase. J Biol Chem 266:20470–20475
Raghothama KG, Karthikeyan AS (2005) Phosphate acquisition. Plant Soil 274:37–49
Römheld V, Müller C, Marschner H (1984) Localization and capacity of proton pumps in roots of intact sunflower plants. Plant Physiol 76:603–606
Sakano K (1990) Proton phosphate stoichiometry in uptake of inorganic-phosphate by cultured-cells of Catharanthus-roseus (L) G-don. Plant Physiol 93:479–483
Sas L, Rengel Z, Tang C (2001) Excess cation uptake, and extrusion of protons and organic acid anions by lupinus albus under phosphorus deficiency. Plant Sci 160:1191–1198
Schubert S (1995) Proton release by plant roots. In: Singh BB, Mengel K (eds) Plant Physiology and Biochemistry. Panina Publishing Corporation, New Delhi, pp 97–119
Sekler I, Pick U (1993) Purification and properties of a plasma membrane H+-ATPase from the extremely acidophilic alga Dunaliella acidophila. Plant Physiol 101:1055–1061
Serrano R (1989) Structure and function of plasma membrane ATPase. Ann Rev Plant Physiol Plant Mol Biol 40:61–94
Shen H, Chen J, Wang Z, Yang C, Sasaki T, Yamamoto Y, Matsumoto H, Yan X (2006) Root plasma membrane H+-ATPase is involved in the adaptation of soybean to phosphorus starvation. J Exp Bot 57:1353–1362
Svennelid F, Olsson A, Piotrowski M, Rosenquist M, Ottman C, Larsson C, Oecking C, Sommarin M (1999) Phosphorylation of Thr-948 at the C terminus of the plasma membrane H+-ATPase creates a binding site for the regulatory 14-3-3 protein. Plant Cell 11:2379–2391
Sze H, Li X, Palmgren MG (1999) Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell 11:677–689
Ullrich-Eberius CI, Novacky A, Fischer E, Lüttge U (1981) Relationship between energy-dependent phosphate uptake and the electrical membrane potential in lemna gibba G1. Plant Physiol 67:797–801
Ullrich WR, Larsson M, Larsson CM, Lesch S, Novacky A (1984) Ammonium uptake in Lemna gibba G1, related membrane potential changes, and inhibition of anion uptake. Physiol Plant 61:369–376
Yamaya T, Oaks A (2004) Metabolic regulation of ammonium uptake and assimilation. In: Stulen I, Smancio S (eds) Nitrogen acquisition and assimilation in higher plants (Plant Ecophysiology Series). Kluwer Academic Publisher, The Netherlands, pp 35–64
Yan F, Feuerle R, Schäffer S, Fortmeier H, Schubert S (1998) Adaptation of active proton pumping and plasmalemma ATPase activity of corn roots to low root medium pH. Plant Physiol 117:311–319
Yan F, Zhu Y, Muller C, Zörb C, Schubert S (2002) Adaptation of H+-pumping and plasma membrane H+ ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol 129:50–63
Zhu Y, Yan F, Zörb C, Schubert S (2005) A link between citrate and porton release by proteoid roots of white lupin (Lupinus albus L.) grown under phosphorus deficient conditions? Plant Cell Physiol 46:892–901
Zhu Y, Di T, Xu G, Chen X, Zeng H, Yan F, Shen Q (2009) Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant Cell Environ 32:1428–1440
Acknowledgements
This work was supported by China 973 Program (2011CB100302), Natural Science Foundation of China (NSFC 30971864) and 111 project (No. B07030). We thank Prof. Uzi Kafkafi for carefully reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: A.C. Borstlap.
Rights and permissions
About this article
Cite this article
Zhang, R., Liu, G., Wu, N. et al. Adaptation of plasma membrane H+ ATPase and H+ pump to P deficiency in rice roots. Plant Soil 349, 3–11 (2011). https://doi.org/10.1007/s11104-011-0774-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0774-2