Abstract
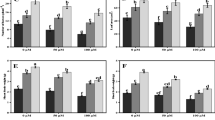
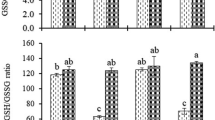
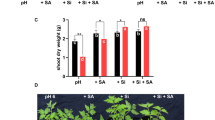
We investigated effect of silicon (Si) on the growth, uptake of sodium (Na), chloride (Cl), boron (B), stomatal resistance (SR), lipid peroxidation (MDA), membrane permeability (MP), lipoxygenase (LOX) activity, proline (PRO) accumulation, H2O2 accumulation, non-enzymatic antioxidant activity (AA) and the activities of major antioxidant enzymes (superoxide dismutase, SOD; catalase, CAT and ascorbate peroxidase, APX) of spinach and tomato grown in sodic-B toxic soil. Si applied to the sodic-B toxic soil at 2.5 and 5.0 mM concentrations significantly increased the Si concentration in the plant species and counteracted the deleterious effects of high concentrations of Na, Cl and B on root and shoot growth by lowering the accumulation of these elements in the plants. Stomatal resistance, MP, MDA and the concentrations of H2O2 and PRO were higher in the plants grown in sodic-B toxic soil without Si: LOX activity of excised leaves of both species was increased by Si. Antioxidant activities of both species were significantly affected by Si, with the activities of SOD, CAT and APX decreased and AA increased by applied Si. For most of the parameters measured, it was found that 5 mM Si was more effective than the 2.5 mM Si. Based on the present work, it can be concluded that Si alleviates sodicity and B toxicity of the plants grown in sodic-B toxic soil by preventing both oxidative membrane damage and also translocation of Na, Cl and B from root to shoots and/or soil to plant, and lowering the phytotoxic effects of Na, Cl and B within plant tissues. It was concluded that tomato was more responsive to Si than spinach since it was more salt sensitive than spinach. To our knowledge, this is the first report that Si improves the combined salt and B tolerance of spinach and tomato grown in naturally sodic-B toxic soil, and which describes membrane-related parameters and antioxidant responses.


Similar content being viewed by others
Abbreviations
- AA:
-
Non-enzymatic antioxidant activity
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- LOX:
-
Lipoxygenase
- MP:
-
Membrane permeability
- MDA:
-
Malondialdehyde
- NBT:
-
Nitroblue tetrazolium
- PRO:
-
Proline
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- SR:
-
Stomatal resistance
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
References
Ahmad R, Zaheer S, Ismail S (1992) Role of silicon in salt tolerance of wheat (Triticum aestivum L.). Plant Sci 85:43–50
Alpaslan M, Gunes A (2001) Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants. Plant Soil 236:123–128
Al-Aghabary K, Zhu Z, Shi Q (2004) Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J Plant Nutr 12:2101–2115
Axelrod B, Cheesbrough TM, Laakso S (1981) Lipoxygenases from soybeans. In: Lowenstein JM (ed) Methods in enzymology. Academic Press, New York, pp 441–451
Bates LS, Waldren RP, Teare JD (1973) Rapid determination of proline for water stress studies. Plant Soil 39:205–207
Ben-Gal A, Shani U (2002) Yield, transpiration and growth of tomatoes under combined excess boron and salinity stress. Plant Soil 247:211–221
Bradbury M, Ahmad R (1990) The effect of silicon on the growth of Prosopis juliflora growing in saline soil. Plant Soil 125:71–74
Cakmak I, Strbac D, Marschner H (1993) Activities of hydrogen peroxide-scavenging enzymes in germinated wheat seeds. J Exp Bot 44:127–132
Epstein E (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol 50:641–664
Garcia POC, Rivero RM, Lopez-Lefebre LR, Sanchez E, Ruiz JM, Romero L (2001) Response of oxidative metabolism to the application of carbendazim plus boron in tobacco. Aust J Plant Physiol 28:801–806
Giannopolitis CN, Ries SK (1977) Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol 59:309–314
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Gunes A, Inal A, Alpaslan M (1996) Effect of salinity on stomatal resistance, proline and mineral composition of pepper. J Plant Nutr 19:389–396
Guo W, Huo YL, Wang SG, Zhu YG (2005) Effect of silicate on the growth and arsenate uptake by rice (Oryza sativa L.) seedlings in solution culture. Plant Soil 272:173–181
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Oxford University Press, UK
Hattori T, Inanaha S, Araki H, An P, Morita S, Luxova M, Lux A (2005) Application of silicon enhanced tolerance in Sorghum bicolor. Physiol Plant 123:459–466
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Inal A, Tarakcioglu C (2001) Effects of nitrogen forms on growth, nitrate accumulation, membrane permeability and nitrogen use efficiency of hydroponically grown bunch onion under boron deficiency and toxicity. J Plant Nutr 24:1521–1534
Ismail AM (2003) Response of maize and sorghum to excess boron and salinity. Biol Plant 42:313–316
Iwasaki K, Maier P, Fecth M, Horst WJ (2002) Leaf apoplastic silicon enhances manganese tolerance of cowpea (Vigna unguiculata). J Plant Physiol 159:167–173
Jain M, Mathur G, Koul S, Sarin NB (2001) Ameliorative effects of proline on salt stressed lipid peroxidation in cell lines of groundnut (Arachis hypogea L.). Plant Cell Rep 20:463–468
Karabal E, Yucel M, Oktem HA (2003) Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci 164:925–933
Keles Y, Oncel I, Yenice N (2004) Relationship between boron content and antioxidant compounds in Citrus leaves taken from field with different water source. Plant Soil 265:345–353
Liang Y (1999) Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil 209:217–224
Liang Y, Shen Z (1994) Interaction of silicon and boron in oilseed rape plants. J Plant Nutr 17:415–425
Liang Y, Wong JWC, Wei L (2005) Silicon mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Ma JF (2004) Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr 50:11–18
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I (2006) Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh). Environ Exp Bot 56:54–62
Morikowa CK, Saigusa M (2002) Si amelioration of Al toxicity in barley (Hordeum vulgare L.) growing in two Andosol. Plant Soil 240:161–168
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Nable RO, Banuelos GS, Paull JG (1997) Boron toxicity. Plant Soil 193:181–198
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Page AL, Miller RH, Keeney DR (1982) Methods of soil analysis, part 2; chemical and microbiological properties, 2nd edn. SSSA, Madison, WI, USA
Papadakis IE, Dimassi KN, Bosabadilis AM, Therios IN, Pataks A, Giannakoula A (2004) Boron toxicity in ‘Clementine’ mandarin plants grafted on two rootstocks. Plant Sci 166:539–547
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Rahnama H, Ebrahimzadeh H (2005) The effect of NaCl on antioxidant enzyme activities in potato seedlings. Biol Plant 49:93–97
Rance I, Fournier J, Esquerre-Tugaye MT (1998) The incompatible interaction between Phytophthora parasitica var. nicotianae race and tobacco is suppressed in transgenic plants expressing antisense lipoxygenase sequences. Proc Natl Acad Sci USA 95:6554–6559
Rogalla H, Römheld V (2002) Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ 25:549–555
Romero-Aranda MR, Jurado O, Cuartero J (2006) Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J Plant Physiol 163:847–855
Sairam RK, Srivastava GC, Agarwal S, Meena RC (2005) Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol Plant 49:85–91
Treder W, Cieslinski G (2005) Effect of silicon application on cadmium uptake and distribution in strawberry plants grown on contaminated soils. J Plant Nutr 28:917–929
Tsai YC, Hong CY, Liu LF, Kao CH (2004) Relative importance of Na and Cl in NaCl-induced antioxidative systems in roots of rice seedlings. Physiol Plant 122:86–94
Van der Vorm PDJ (1987) Dry ashing of plant material and dissolution of the ash in HF for the colorimetric determination of silicon. Commun Soil Sci Plant Anal 18:1181–1189
Wolf B (1971) The determination of boron in soil extracts, plant materials, composts, manures, water and nutrient solutions. Commun Soil Sci Plant Anal 2:363–374
Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant response to osmotic stress. Plant Cell Environ 25:131–139
Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci 10:615–620
Yan B, Dai Q, Liu X, Huang S, Wang Z (1996) Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 179:261–268
Yeo AR, Flowers SA, Rao G, Welfare K, Senanayake N, Flowers TJ (1999) Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for a reduction in the transpirational bypass flow. Plant Cell Environ 22:559–565
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167:527–533
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunes, A., Inal, A., Bagci, E.G. et al. Silicon-mediated changes of some physiological and enzymatic parameters symptomatic for oxidative stress in spinach and tomato grown in sodic-B toxic soil. Plant Soil 290, 103–114 (2007). https://doi.org/10.1007/s11104-006-9137-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9137-9