Abstract
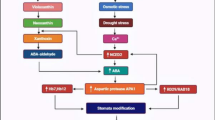
Phytocystatins are well-known inhibitors of C1A cysteine proteinases. However, previous research has revealed legumain (C13) protease inhibition via a carboxy-extended phytocystatin. Among the 12 phytocystatins genes in rice, OcXII is the only gene possessing this carboxy-terminal extension. The specific legumain inhibition activity was confirmed, in our work, using a recombinant OcXII harboring only the carboxy-terminal domain and this part did not exhibit any effect on papain-like activities. Meanwhile, rice plants silenced at the whole OcXII gene presented higher legumain and papain-like proteolytic activities, resulting in a faster initial seedling growth. However, when germinated under stressful alkaline conditions, OcXII-silenced plants exhibited impaired root formation and delayed shoot growth. Interestingly, the activity of OcXII promoter gene was detected in the rice seed scutellum region, and decreases with seedling growth. Seeds from these plants also exhibited slower growth at germination under ABA or alkaline conditions, while maintaining very high levels of OcXII transcriptional activation. This likely reinforces the proteolytic control necessary for seed germination and growth. In addition, increased legumain activity was detected in OcXII RNAi plants subjected to a fungal elicitor. Overall, the results of this study highlight the association of OcXII with not only plant development processes, but also with stress response pathways. The results of this study reinforce the bifunctional ability of carboxy-extended phytocystatins in regulating legumain proteases via its carboxy-extended domain and papain-like proteases by its amino-terminal domain.








Similar content being viewed by others
References
Abe K, Emori Y, Kondo H, Arai S, Suzuki K (1988) The NH2-terminal 21 amino acid residues are not essential for the papain-inhibitory activity of oryzacystatin, a member of the cystatin superfamily. J Biol Chem 263:7655–7659
Agrawal GK, Rakwal R, Tamogami S, Yonekura M, Akihiro K, Hikaru S (2002) Chitosan activates defense/stress response (s) in the leaves of Oryza sativa seedlings. Plant Physiol Biochem 40:1061–1069
Alvarez-Fernandez M, Barrett a J, Gerhartz B, Dando PM, Ni J, Abrahamson M (1999) Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J Biol Chem 274:19195–19203
Arai S, Watanabe H, Kondo H (1991) Papain activity of oryzacystatin, a rice seed cysteine proteinase inhibitor, depends on the central Gln–Val–Val–Ala–Gly region conserved among cystatin superfamily members. J Biochem 109:294–298
Arai S, Matsumoto I, Emori Y, Abe K (2002) Plant seed cystatins and their target enzymes of endogenous and exogenous origin. J Agric Food Chem 50:6612–6617
Belenghi B, Acconcia F, Trovato M, Perazzolli M, Bocedi A, Polticelli F, Ascenzi P, Delledonne M (2003) AtCYS1, a cystatin from Arabidopsis thaliana, suppresses hypersensitive cell death. Eur J Biochem 270:2593–2604. doi:10.1046/j.1432-1033.2003.03630.x
Benchabane M, Schlüter U, Vorster J, Goulet M-C, Michaud D (2010) Plant cystatins. Biochimie 92:1657–1666. doi:10.1016/j.biochi.2010.06.006
Berman HM (2000) The protein data bank. Nucleic Acids Res 28:235–242. doi:10.1093/nar/28.1.235
Bode W, Engh R, Musil D, Thiele U, Huber R, Karshikov A, Brzin J, Kos J, Turk V (1988) The 2.0 A X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J 7:2593
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bustin S, Benes V, Garson J et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. doi:10.1373/clinchem.2008.112797
Cao P, Jung K-H, Choi D, Hwang D, Zhu J, Ronald PC (2012) The rice oligonucleotide array database: an atlas of rice gene expression. Rice 5:17. doi:10.1186/1939-8433-5-17
Carrillo L, Herrero I, Cambra I, Sánchez-Monge R, Diaz I, Martinez M (2011a) Differential in vitro and in vivo effect of barley cysteine and serine protease inhibitors on phytopathogenic microorganisms. Plant Physiol Biochem 1–10. doi:10.1016/j.plaphy.2011.03.012
Carrillo L, Martinez M, Ramessar K, Cambra I, Castañera P, Ortego F, Díaz I (2011b) Expression of a barley cystatin gene in maize enhances resistance against phytophagous mites by altering their cysteine–proteases. Plant Cell Rep 30:101–112. doi:10.1007/s00299-010-0948-z
Chang W-C, Lee T-Y, Huang H-D, Huang H-Y, Pan R-L (2008) PlantPAN: plant promoter analysis navigator, for identifying combinatorial cis-regulatory elements with distance constraint in plant gene groups. BMC Genomics 9:561. doi:10.1186/1471-2164-9-561
Cheng M-L, Tzen JTC, Shyu DJH, Chou W-M (2014) Functional characterization of the N-terminal and C-terminal domains of a sesame group II phytocystatin. Bot Stud 55:18. doi:10.1186/1999-3110-55-18
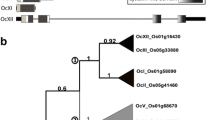
Christoff AP, Margis R (2014) The diversity of rice phytocystatins. Mol Genet Genomics 289:1321–1330. doi:10.1007/s00438-014-0892-7
Christoff AP, Turchetto-Zolet AC, Margis R (2014) Uncovering legumain genes in rice. Plant Sci 215–216:100–109. doi:10.1016/j.plantsci.2013.11.005
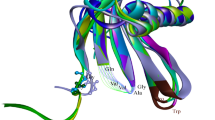
Chu M-H, Liu K-L, Wu H-Y, Yeh K-W, Cheng Y-S (2011) Crystal structure of tarocystatin-papain complex: implications for the inhibition property of group-2 phytocystatins. Planta 234:243–254. doi:10.1007/s00425-011-1398-8
Diaz-Mendoza M, Velasco-Arroyo B, Gonzalez-Melendi P, Martinez M, Diaz I (2014) C1A cysteine protease–cystatin interactions in leaf senescence. J Exp Bot. doi:10.1093/jxb/eru043
Dutt S, Singh VK, Marla SS, Kumar A (2010) In silico analysis of sequential, structural and functional diversity of wheat cystatins and its implication in plant defense. Genomics Proteomics Bioinforma Beijing Genomics Inst 8:42–56. doi:10.1016/S1672-0229(10)60005-8
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS (2011) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 22:1–9. doi:10.1093/nar/gkr944
Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura I (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305:855–858. doi:10.1126/science.1099859
Hatsugai N, Yamada K, Goto-Yamada S, Hara-Nishimura I (2015) Vacuolar processing enzyme in plant programmed cell death. Front Plant Sci 6:1–11. doi:10.3389/fpls.2015.00234
Hong JK, Hwang JE, Lim CJ, Yang KA, Jin Z-L, Kim CY, Koo JC, Chung WS, Lee KO, Lee SY, Cho MJ, Lim CO (2007) Over-expression of Chinese cabbage phytocystatin 1 retards seed germination in Arabidopsis. Plant Sci 172:556–563. doi:10.1016/j.plantsci.2006.11.005
Hong JK, Hwang JE, Chung WS, Lee KO, Choi YJ, Gal SW, Park BS, Lim CO (2008) Expression of a Chinese cabbage cysteine proteinase inhibitor, BrCYS1, retards seed germination and plant growth in transgenic tobacco plant. J Plant Biol 51:347–353
Hwang JE, Hong JK, Je JH, Lee KO, Kim DY, Lee SY, Lim CO (2009) Regulation of seed germination and seedling growth by an Arabidopsis phytocystatin isoform, AtCYS6. Plant Cell Rep 28:1623–1632. doi:10.1007/s00299-009-0762-7
Hwang JE, Hong JK, Lim CJ, Chen H, Je J, Yang KA, Kim DY, Choi YJ, Lee SY, Lim CO (2010) Distinct expression patterns of two Arabidopsis phytocystatin genes, AtCYS1 and AtCYS2, during development and abiotic stresses. Plant Cell Rep 29:905–915. doi:10.1007/s00299-010-0876-y
Jefferson R a, Kavanagh T a, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Karimi M, Inzé D, Depicker A (2002) GATEWAY™ vectors for agrobacterium-mediated plant transformation. Trends Plant Sci 7:193–195
Kato H, Minamikawa T (1996) Identification and characterization of a rice cysteine endopeptidase that digests glutelin. Eur J Biochem 239:310–316
Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi:10.1038/nprot.2015.053
Kunert KJ, van Wyk SG, Cullis C a., Vorster BJ, Foyer CH (2015) Potential use of phytocystatins in crop improvement, with a particular focus on legumes. J Exp Bot. doi:10.1093/jxb/erv211
Lepelley M, Amor M Ben, Martineau N, Cheminade G, Caillet V, McCarthy J (2012) Coffee cysteine proteinases and related inhibitors with high expression during grain maturation and germination. BMC Plant Biol 12:31. doi:10.1186/1471-2229-12-31
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Lv BS, Li XW, Ma HY, Sun Y, Wei LX, Jiang CJ, Liang ZW (2013) Differences in growth and physiology of rice in response to different saline-alkaline stress factors. Agron J 105:1119–1128. doi:10.2134/agronj2013.0017
Margis R, Reis EM, Villeret V (1998) Structural and phylogenetic relationships among plant and animal cystatins. Arch Biochem Biophys 359:24–30. doi:10.1006/abbi.1998.0875
Margis-Pinheiro M, Zolet ACT, Loss G, Pasquali G, Margis R (2008) Molecular evolution and diversification of plant cysteine proteinase inhibitors: new insights after the poplar genome. Mol Phylogenet Evol 49:349–355. doi:10.1016/j.ympev.2008.04.025
Martinez M, Abraham Z, Gambardella M, Echaide M, Carbonero P, Diaz I (2005) The strawberry gene Cyf1 encodes a phytocystatin with antifungal properties. J Exp Bot 56:1821–1829. doi:10.1093/jxb/eri172
Martinez M, Diaz-Mendoza M, Carrillo L, Diaz I (2007) Carboxy terminal extended phytocystatins are bifunctional inhibitors of papain and legumain cysteine proteinases. FEBS Lett 581:2914–2918. doi:10.1016/j.febslet.2007.05.042
Martinez M, Cambra I, Carrillo L, Diaz-Mendoza M, Diaz I (2009) Characterization of the entire cystatin gene family in barley and their target cathepsin l-like cysteine-proteases, partners in the hordein mobilization during seed germination. Plant Physiol 151:1531–1545. doi:10.1104/pp.109.146019
Martínez M, Abraham Z, Carbonero P, Díaz I (2005) Comparative phylogenetic analysis of cystatin gene families from arabidopsis, rice and barley. Mol Genet Genomics 273:423–432. doi:10.1007/s00438-005-1147-4
Megdiche W, Passaquet C, Zourrig W, Zuily Fodil Y, Abdelly C (2009) Molecular cloning and characterization of novel cystatin gene in leaves Cakile maritima halophyte. J Plant Physiol 166:739–749. doi:10.1016/j.jplph.2008.09.012
Miki D, Shimamoto K (2004) Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol 45:490–495
Mikkonen A, Porali I, Cercos M, Ho TH (1996) A major cysteine proteinase, EPB, in germinating barley seeds: structure of two intronless genes and regulation of expression. Plant Mol Biol 31:239–254
Müntz K, Blattner FR, Shutov AD (2002) Legumains—a family of asparagine-specific cysteine endopeptidases involved in propolypeptide processing and protein breakdown in plants. J Plant Physiol 159:1281–1293
Nicholas KB, Nicholas HBJ (1997) GeneDoc: a tool for editing and annotating multiple sequence alignments
Novinec M, Lenarčič B (2013) Papain-like peptidases: structure, function, and evolution. Biomol Concepts 4:287–308. doi:10.1515/bmc-2012-0054
Ohtsubo S, Kobayashi H, Noro W, Taniguchi M, Saitoh E (2005) Molecular cloning and characterization of oryzacystatin-III, a novel member of phytocystatin in rice (Oryza sativa L. japonica). J Agric Food Chem 53:5218–5224. doi:10.1021/jf050348j
Okamoto T, Yuki a, Mitsuhashi N, Minamikawa T, Mimamikawa T (1999) Asparaginyl endopeptidase (VmPE-1) and autocatalytic processing synergistically activate the vacuolar cysteine proteinase (SH-EP). Eur J Biochem 264:223–232. doi:10.1046/j.1432-1327.1999.00618.x
Pesquet E (2012) Plant proteases-from detection to function. Physiol Plant 145:1–4. doi:10.1111/j.1399-3054.2012.01614.x
Pierre O, Hopkins J, Combier M, Baldacci F, Engler G, Brouquisse R, Hérouart D, Boncompagni E (2014) Involvement of papain and legumain proteinase in the senescence process of Medicago truncatula nodules. New Phytol 202:849–863. doi:10.1111/nph.12717
Pirovani CP, da Silva Santiago A, Dos Santos LS, Micheli F, Margis R, da Silva Gesteira A, Alvim FC, Pereira GAG, de Mattos Cascardo JC (2010) Theobroma cacao cystatins impair Moniliophthora perniciosa mycelial growth and are involved in postponing cell death symptoms. Planta 232:1485–1497. doi:10.1007/s00425-010-1272-0
Qiang X, Zechmann B, Reitz MU, Kogel K-H, Schäfer P (2012) The mutualistic fungus Piriformospora indica colonizes Arabidopsis roots by inducing an endoplasmic reticulum stress-triggered caspase-dependent cell death. Plant Cell 24:794–809. doi:10.1105/tpc.111.093260
Quain MD, Makgopa ME, Márquez-García B, Comadira G, Fernandez-Garcia N, Olmos E, Schnaubelt D, Kunert KJ, Foyer CH (2014) Ectopic phytocystatin expression leads to enhanced drought stress tolerance in soybean (Glycine max) and Arabidopsis thaliana through effects on strigolactone pathways and can also result in improved seed traits. Plant Biotechnol J. doi:10.1111/pbi.12193
Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, Moorman a FM (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37:e45. doi:10.1093/nar/gkp045
Senthilkumar R, Cheng C-P, Yeh K-W (2010) Genetically pyramiding protease-inhibitor genes for dual broad-spectrum resistance against insect and phytopathogens in transgenic tobacco. Plant Biotechnol J 8:65–75. doi:10.1111/j.1467-7652.2009.00466.x
Sun X, Yang S, Sun M, Wang S, Ding X, Zhu D, Ji W, Cai H, Zhao C, Wang X, Zhu Y (2014) A novel Glycine soja cysteine proteinase inhibitor GsCPI14, interacting with the calcium/calmodulin-binding receptor-like kinase GsCBRLK, regulated plant tolerance to alkali stress. Plant Mol Biol 85:33–48. doi:10.1007/s11103-013-0167-4
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Upadhyaya N, Zhou X, Zhu Q, Eamens A, Wang M, Waterhouse P, Dennis E (2002) Transgenic rice. In: O’Brien L, Henry RJ (eds) Transgenic cereals. AACC, Minnesota, pp 28–87
Valdés-Rodríguez S, Guerrero-Rangel A, Melgoza-Villagómez C, Chagolla-López A, Delgado-Vargas F, Martínez-Gallardo N, Sánchez-Hernández C, Délano-Frier J (2007) Cloning of a cDNA encoding a cystatin from grain amaranth (Amaranthus hypochondriacus) showing a tissue-specific expression that is modified by germination and abiotic stress. Plant Physiol Biochem. 45:790–798. doi:10.1016/j.plaphy.2007.07.007
van der Hoorn RAL (2008) Plant proteases: from phenotypes to molecular mechanisms. Annu Rev Plant Biol 59:191–223. doi:10.1146/annurev.arplant.59.032607.092835
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Wang K-M, Kumar S, Cheng Y-S, Venkatagiri S, Yang A-H, Yeh K-W (2008) Characterization of inhibitory mechanism and antifungal activity between group-1 and group-2 phytocystatins from taro (Colocasia esculenta). FEBS J 275:4980–4989. doi:10.1111/j.1742-4658.2008.06631.x
Watanabe H, Abe K, Emori Y, Hosoyama H (1991) Molecular cloning and gibberellin-induced expression of multiple cysteine proteinases of rice seeds (oryzains). J Biol 266:16897–16902
Yang AH, Yeh KW (2005) Molecular cloning, recombinant gene expression, and antifungal activity of cystatin from taro (Colocasia esculenta cv. Kaosiung no. 1). Planta 221:493–501. doi:10.1007/s00425-004-1462-8
Zhang N, Jones BL (1996) Purification and partial characterization of a 31-kDa cysteine endopeptidase from germinated barley. Planta 199:565–572
Zhang X, Liu S, Takano T (2008) Two cysteine proteinase inhibitors from Arabidopsis thaliana, AtCYSa and AtCYSb, increasing the salt, drought, oxidation and cold tolerance. Plant Mol Biol 68:131–143. doi:10.1007/s11103-008-9357-x
Acknowledgments
This work was supported by a CNPq Grant 478417/2012-8. R. Margis received a research fellowship 307868/2011-7 from CNPq and A.P. Christoff a CAPES PhD fellowship. The authors also would like to thank A. Caverzan, Oliveira L.F.V, D.J. Messeder, F.M. Bianchi, and N.E. Junqueira for experimental support.
Author contributions
A.P.C. and R.M designed the research, analyzed the data and wrote the paper; A.P.C performed the experiments; A.P.C and G.P performed plant transformation experiments; A.P.C and C.S. performed enzymatic assays experiments, M.M-P and M.A-F helped with the experimental structure and reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Christoff, A.P., Passaia, G., Salvati, C. et al. Rice bifunctional phytocystatin is a dual modulator of legumain and papain-like proteases. Plant Mol Biol 92, 193–207 (2016). https://doi.org/10.1007/s11103-016-0504-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-016-0504-5