Abstract
Context
Glucocorticoid (GC) exposure increases food intake, but the mechanisms in humans are not known. Investigation of appetite and food craving has not been done in patients with chronic GC exposure due to Cushing’s disease (CD), either before or after treatment, and could provide insight into mechanisms of food intake and obesity in these patients.
Purpose
To examine whether surgical remission of CD changes appetite (prospective consumption, hunger, satisfaction, and fullness) and food cravings (sweet, salty, fatty, and savory); and to identify predictors of appetite and craving in CD remission.
Methods
30 CD patients, mean age 40.0 years (range 17–70), mean BMI 32.3 ± 6.4, were prospectively studied before and at a mean of 17.4 mo. after remission. At each visit fasting and post-test meal (50 % carbohydrate, 35 % protein, 15 % fat) appetite and craving scores were assessed.
Results
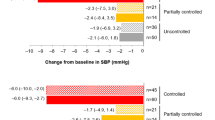
Remission decreased prospective consumption, sweet and savory craving (p < 0.05), but did not change hunger, satisfaction, fullness, or fat craving, despite decreases in BMI and fat mass. In CD remission, serum cortisol predicted lower satisfaction and fullness, and masses of abdominal fat depots predicted higher hunger and consumption (p < 0.05).
Conclusions
Chronic GC exposure in CD patients may stimulate the drive to eat by enhancing craving, rather than regulating the sensation of hunger. Continued alterations in appetite regulation due to abdominal fat mass and circulating cortisol could play a role in the cardiovascular and metabolic risk that has been reported in CD patients despite remission.


Similar content being viewed by others
References
Mayo-Smith W et al (1989) Body fat distribution measured with CT: correlations in healthy subjects, patients with anorexia nervosa, and patients with Cushing syndrome. Radiology 170(2):515–518
Garrapa GG et al (2001) Body composition and metabolic features in women with adrenal incidentaloma or Cushing’s syndrome. J Clin Endocrinol Metab 86(11):5301–5306
Schafroth U et al (2000) Leptin levels in relation to body composition and insulin concentration in patients with endogenous Cushing’s syndrome compared to controls matched for body mass index. J Endocrinol Invest 23(6):349–355
Wajchenberg BL et al (1995) Estimation of body fat and lean tissue distribution by dual energy X-ray absorptiometry and abdominal body fat evaluation by computed tomography in Cushing’s disease. J Clin Endocrinol Metab 80(9):2791–2794
Geer EB et al (2010) MRI assessment of lean and adipose tissue distribution in female patients with Cushing’s disease. Clin Endocrinol 73(4):469–475
Geer EB, et al (2012) Body composition and cardiovascular risk markers after remission of Cushing’s disease: a prospective study using whole-body MRI. J Clin Endocrinol Metab 97(5):1702–1711
Nakayama S et al (2011) Corticotropin-releasing hormone (CRH) transgenic mice display hyperphagia with increased Agouti-related protein mRNA in the hypothalamic arcuate nucleus. Endocr J 58(4):279–286
Zakrzewska KE et al (1999) Induction of obesity and hyperleptinemia by central glucocorticoid infusion in the rat. Diabetes 48(2):365–370
Tataranni PA et al (1996) Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol 271(2 Pt 1):E317–E325
Udden J et al (2003) Effects of glucocorticoids on leptin levels and eating behaviour in women. J Intern Med 253(2):225–231
Colao A et al (1999) Persistence of increased cardiovascular risk in patients with Cushing’s disease after five years of successful cure. J Clin Endocrinol Metab 84(8):2664–2672
Barahona MJ et al (2009) Persistent body fat mass and inflammatory marker increases after long-term cure of Cushing’s syndrome. J Clin Endocrinol Metab 94(9):3365–3371
Faggiano A et al (2003) Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing’s disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab 88(6):2527–2533
Neary NM et al (2013) Hypercortisolism is associated with increased coronary arterial atherosclerosis: analysis of noninvasive coronary angiography using multidetector computerized tomography. J Clin Endocrinol Metab 98(5):2045–2052
Wagenmakers M et al (2015) Persistent centripetal fat distribution and metabolic abnormalities in patients in long-term remission of Cushing’s syndrome. Clin Endocrinol (Oxf) 82(2):180–187
Epel E et al (2001) Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26(1):37–49
Biller BM et al (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93(7):2454–2462
Flint A et al (2000) Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 24(1):38–48
Korner J et al (2006) Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity 14(9):1553–1561
Matthews DR et al (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Snyder WS et al (1975) Report of the task group on reference man, in international commission on radiological protection, no. 23. Pergamon Press, Oxford
Rieth N et al (2009) Effects of short-term corticoid ingestion on food intake and adipokines in healthy recreationally trained men. Eur J Appl Physiol 105(2):309–313
Pecoraro N, Gomez F, Dallman MF (2005) Glucocorticoids dose-dependently remodel energy stores and amplify incentive relativity effects. Psychoneuroendocrinology 30(9):815–825
White BD et al (1994) Type II corticosteroid receptor stimulation increases NPY gene expression in basomedial hypothalamus of rats. Am J Physiol 266(5 Pt 2):R1523–R1529
Stephens TW et al (1995) The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377(6549):530–532
Keim NL, Stern JS, Havel PJ (1998) Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr 68(4):794–801
Doucet E et al (2000) Appetite after weight loss by energy restriction and a low-fat diet-exercise follow-up. Int J Obes Relat Metab Disord 24(7):906–914
Tomiyama AJ, Dallman MF, Epel ES (2011) Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology 36(10):1513–1519
Kojima M et al (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402(6762):656–660
Date Y et al (2000) Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141(11):4255–4261
Cummings DE et al (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50(8):1714–1719
Batterham RL et al (2003) Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med 349(10):941–948
Batterham RL et al (2002) Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418(6898):650–654
Degen L et al (2005) Effect of peptide YY3-36 on food intake in humans. Gastroenterology 129(5):1430–1436
Moran TH et al (2005) Peptide YY(3-36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 288(2):R384–R388
Koegler FH et al (2005) Peptide YY(3-36) inhibits morning, but not evening, food intake and decreases body weight in rhesus macaques. Diabetes 54(11):3198–3204
Otto B et al (2004) Endogenous and exogenous glucocorticoids decrease plasma ghrelin in humans. Eur J Endocrinol 151(1):113–117
Libe R et al (2005) Ghrelin and adiponectin in patients with Cushing’s disease before and after successful transsphenoidal surgery. Clin Endocrinol 62(1):30–36
Kageyama K et al (2012) Dexamethasone stimulates the expression of ghrelin and its receptor in rat hypothalamic 4B cells. Regul Pept 174(1–3):12–17
Lam TK et al (2005) Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11(3):320–327
Acknowledgments
The authors wish to thank the individuals who volunteered to participate in this study, the staff at the Mount Sinai Clinical Research Unit, Mark Punyanitya at the Image Reading Center, and Marie Grace at the Mount Sinai Clinical laboratory. Supported by National Institutes of Health Grant K23 DK 082617 and Mount Sinai General Clinical Research Center CReFF award MO1-RR-00071 to EBG, Grant UL1TR000067 to the Mount Sinai CTSA, Grant TL1RR029886 from the National Center for Research Resources, and Grant K24 DK 073040 to PUF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant disclosures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
Mean fasting and post-meal total ghrelin and PYY concentrations in 18 CD patients before and after surgical remission. Surgical remission increases mean fasting and post-meal total ghrelin (A) but not PYY (B) concentrations. Supplementary material 1 (XLSX 22 kb)
Rights and permissions
About this article
Cite this article
Geer, E.B., Lalazar, Y., Couto, L.M. et al. A prospective study of appetite and food craving in 30 patients with Cushing’s disease. Pituitary 19, 117–126 (2016). https://doi.org/10.1007/s11102-015-0690-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-015-0690-1