Purpose
α-Internexin (INA) is a class IV neuronal intermediate filament protein that maintains the morphogenesis of neurons. It is expressed in developing neuroblasts and represents the major component of the cytoskeleton in cerebellar granule cells of adult central nervous system tissue. Data concerning INA expression in the human frontal pituitary lobe and related adenomas (PA) is missing.
Methods
Using immunohistochemistry we examined the distribution pattern of INA in a large cohort of 152 PA, 11 atypical PA, 4 pituitary carcinomas and 20 normal pituitaries (overall n = 187). Quantity of INA protein expression was semi-quantitatively evaluated and grouped into five categories (0 = 0 %; 1 = >0–5 %; 2 = >5–35 %; 3 = >35–80 %; 4 = >80 % of cells).
Results
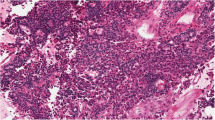
Cellular staining intensity of INA appeared significantly higher in gonadotropinomas (Go, n = 62), null cell adenomas (NC, n = 7) and thyrotropinomas (TSHomas, n = 7) compared to the other tumor subtypes (p ≤ 0.001). Furthermore, Go and NC showed a peculiar pseudorosette-like staining pattern surrounding blood vessels in 85.5 % (59/69) of cases. Interestingly, areas exhibiting homogenous INA staining were often associated with oncocytic cell changes and decreased immunohistochemically detectable hormone expression. Only 8.5 % (8/94) of other PA showed a comparable INA distribution (p ≤ 0.001).
Conclusion
Go, NC as well as TSHomas exhibit high levels of intracellular INA protein indicating neuronal transdifferentiation. A possible impact on pathogenesis and endocrine activity needs further investigation.


Similar content being viewed by others
References
Ezzat S, Asa SL (2006) Mechanisms of disease: the pathogenesis of pituitary tumors. Nat Clin Pract Endocrinol Metab 2(4):220–230
Ezzat S et al (2004) The prevalence of pituitary adenomas: a systematic review. Cancer 101(3):613–619
DeLellis RA, Lloyd RV, Heitz PU, Eng C (2004) Tumours of endocrine organs. IARC, Lyons
Asa SL, Ezzat S (2009) The pathogenesis of pituitary tumors. Annu Rev Pathol 4:97–126
Asa SL (1998) Tumors of the pituitary gland. In: Rosai J, Sobin HL (eds) Atlas of tumor pathology. AFIP, Washington, DC
Melmed S (2003) Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Investig 112(11):1603–1618
Abbass SA, Asa SL, Ezzat S (1997) Altered expression of fibroblast growth factor receptors in human pituitary adenomas. J Clin Endocrinol Metab 82(4):1160–1166
Zhu X et al (2007) Epigenetic silencing through DNA and histone methylation of fibroblast growth factor receptor 2 in neoplastic pituitary cells. Am J Pathol 170(5):1618–1628
Zhou Y, Zhang X, Klibanski A (2014) Genetic and epigenetic mutations of tumor suppressive genes in sporadic pituitary adenoma. Mol Cell Endocrinol 386(1–2):16–33
Pachter JS, Liem RK (1985) Alpha-internexin, a 66-kD intermediate filament-binding protein from mammalian central nervous tissues. J Cell Biol 101(4):1316–1322
Kaplan MP et al (1990) Alpha-internexin, a novel neuronal intermediate filament protein, precedes the low molecular weight neurofilament protein (NF-L) in the developing rat brain. J Neurosci 10(8):2735–2748
Cooper GM (2000) The cell. A molecular approach, 2nd edn. Sinauer Associates, Sunderland, MA
Ho CL, Liem RK (1996) Intermediate filaments in the nervous system: implications in cancer. Cancer Metastasis Rev 15(4):483–497
Eaker EY, Sallustio JE (1994) The distribution of novel intermediate filament proteins defines subpopulations of myenteric neurons in rat intestine. Gastroenterology 107(3):666–674
Krammer HJ et al (1994) Immunohistochemistry of markers of the enteric nervous system in whole-mount preparations of the human colon. Eur J Pediatr Surg 4(5):274–278
Schimmack S et al (2012) The clinical implications and biologic relevance of neurofilament expression in gastroenteropancreatic neuroendocrine neoplasms. Cancer 118(10):2763–2775
Ishida M et al (2008) Co-expression of neuronal intermediate filaments, peripherin and alpha-internexin in human well-differentiated endocrine neoplasms (carcinoid tumors) of the appendix. Mol Med Rep 1(2):191–195
Fliegner KH et al (1994) Expression of the gene for the neuronal intermediate filament protein alpha-internexin coincides with the onset of neuronal differentiation in the developing rat nervous system. J Comp Neurol 342(2):161–173
Chien CL, Liem RK (1995) The neuronal intermediate filament, alpha-internexin is transiently expressed in amacrine cells in the developing mouse retina. Exp Eye Res 61(6):749–756
Chien CL, Mason CA, Liem RK (1996) Alpha-internexin is the only neuronal intermediate filament expressed in developing cerebellar granule neurons. J Neurobiol 29(3):304–318
Helfand BT et al (2003) A role for intermediate filaments in determining and maintaining the shape of nerve cells. Mol Biol Cell 14(12):5069–5081
Ching GY, Liem RK (1999) Analysis of the roles of the head domains of type IV rat neuronal intermediate filament proteins in filament assembly using domain-swapped chimeric proteins. J Cell Sci 112(Pt 13):2233–2240
Ching GY, Liem RK (1998) Roles of head and tail domains in alpha-internexin’s self-assembly and coassembly with the neurofilament triplet proteins. J Cell Sci 111(Pt 3):321–333
Chien CL et al (2005) Overexpression of neuronal intermediate filament protein alpha-internexin in PC12 cells. J Neurosci Res 80(5):693–706
Ohara O et al (1993) Neurofilament deficiency in quail caused by nonsense mutation in neurofilament-L gene. J Cell Biol 121(2):387–395
Liem RK, Messing A (2009) Dysfunctions of neuronal and glial intermediate filaments in disease. J Clin Investig 119(7):1814–1824
Lu XY et al (2010) Anti-alpha-internexin autoantibody from neuropsychiatric lupus induce cognitive damage via inhibiting axonal elongation and promote neuron apoptosis. PLoS One 5(6):e11124
Reddy TR et al (1998) Specific interaction of HTLV tax protein and a human type IV neuronal intermediate filament protein. Proc Natl Acad Sci USA 95(2):702–707
Ikota H et al (2006) Systematic immunohistochemical profiling of 378 brain tumors with 37 antibodies using tissue microarray technology. Acta Neuropathol 111(5):475–482
Ducray F et al (2011) Diagnostic and prognostic value of alpha internexin expression in a series of 409 gliomas. Eur J Cancer 47(5):802–808
Eigenbrod S et al (2011) Alpha-internexin in the diagnosis of oligodendroglial tumors and association with 1p/19q status. J Neuropathol Exp Neurol 70(11):970–978
Nagaishi M et al (2013) Alpha-internexin and altered CIC expression as a supportive diagnostic marker for oligodendroglial tumors with the 1p/19q co-deletion. Brain Tumor Pathol. doi:10.1007/s10014-013-0168-7
Mokhtari K et al (2011) Alpha-internexin expression predicts outcome in anaplastic oligodendroglial tumors and may positively impact the efficacy of chemotherapy: European organization for research and treatment of cancer trial 26951. Cancer 117(13):3014–3026
Willoughby V et al (2008) A comparative immunohistochemical analysis of small round cell tumors of childhood: utility of peripherin and alpha-internexin as markers for neuroblastomas. Appl Immunohistochem Mol Morphol 16(4):344–348
Cairns NJ et al (2004) Alpha-internexin aggregates are abundant in neuronal intermediate filament inclusion disease (NIFID) but rare in other neurodegenerative diseases. Acta Neuropathol 108(3):213–223
Kimura A et al (2008) Proteomic analysis of autoantibodies in neuropsychiatric systemic lupus erythematosus patient with white matter hyperintensities on brain MRI. Lupus 17(1):16–20
Rajasalu T et al (2004) Demonstration of natural autoantibodies against the neurofilament protein alpha-internexin in sera of patients with endocrine autoimmunity and healthy individuals. Immunol Lett 94(1–2):153–160
Ching GY et al (1999) Overexpression of alpha-internexin causes abnormal neurofilamentous accumulations and motor coordination deficits in transgenic mice. J Neurosci 19(8):2974–2986
Buslei R et al (2007) Nuclear beta-catenin accumulation associates with epithelial morphogenesis in craniopharyngiomas. Acta Neuropathol 113(5):585–590
Horvath E, Kovacs K (1978) Morphogenesis and significance of fibrous bodies in human pituitary adenomas. Virchows Arch B Cell Pathol 27(1):69–78
Kawamura K, Kikuyama S (1998) Morphogenesis of the hypothalamus and hypophysis: their association, dissociation and reassociation before and after “Rathke”. Arch Histol Cytol 61(3):189–198
Zhu X, Gleiberman AS, Rosenfeld MG (2007) Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87(3):933–963
Ju G (1997) Innervation of the mammalian anterior pituitary: a mini review. Microsc Res Tech 39(2):131–137
Liu S (2004) Peptidergic innervation in pars distalis of the human anterior pituitary. Brain Res 1008(1):61–68
Martinez-Campos A, Dannies PS (1986) A possible differentiation of anterior pituitary cells in collagen gels into neurons. Cell Tissue Res 244(1):21–26
Horvath E et al (1994) Pituitary adenoma with neuronal choristoma (PANCH): composite lesion or lineage infidelity? Ultrastruct Pathol 18(6):565–574
Missale C et al (1996) Nerve growth factor in the anterior pituitary: localization in mammotroph cells and cosecretion with prolactin by a dopamine-regulated mechanism. Proc Natl Acad Sci USA 93(9):4240–4245
Scheithauer BW et al (1999) Prolactin-producing pituitary adenoma and carcinoma with neuronal components—a metaplastic lesion. Pituitary 1(3–4):197–205
Chen J et al (2005) The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 146(9):3985–3998
Kontogeorgos G et al (2006) Ganglion cell containing pituitary adenomas: signs of neuronal differentiation in adenoma cells. Acta Neuropathol 112(1):21–28
Johnson MD et al (2007) Neuronal differentiation and expression of neural epitopes in pituitary adenomas. J Histochem Cytochem 55(12):1265–1271
Ho DM et al (1997) The clinicopathological characteristics of gonadotroph cell adenoma: a study of 118 cases. Hum Pathol 28(8):905–911
Niveiro M et al (2004) Oncocytic transformation in pituitary adenomas: immunohistochemical analyses of 65 cases. Arch Pathol Lab Med 128(7):776–780
Lindner J et al (1990) Regulation of pituitary glycoprotein alpha-subunit secretion after administration of a luteinizing hormone-releasing hormone antagonist in normal men. J Clin Endocrinol Metab 70(4):1219–1224
Dasen JS et al (1999) Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell 97(5):587–598
Liu B et al (2014) Alpha-internexin: a novel biomarker for pancreatic neuroendocrine tumor aggressiveness. J Clin Endocrinol Metab 99(5):E786–E795. doi:10.1210/jc.2013-2874
Trojanowski JQ, Lee VM, Schlaepfer WW (1984) An immunohistochemical study of human central and peripheral nervous system tumors, using monoclonal antibodies against neurofilaments and glial filaments. Hum Pathol 15(3):248–257
Acknowledgments
We thank T. Jungbauer and D. Maron for expert technical assistance. We also thank A. Schult for carefully reading and discussing the manuscript. The study was funded by the Dr. Ernst und Anita Bauer Stiftung, Nuremberg, Germany. The present work was performed in partial fulfillment of the requirements for obtaining the degree “Dr. Med.” at the Friedrich-Alexander University Erlangen-Nürnberg (FAU), Germany.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All patients had given their informed consent for processing the specimens. The study was approved by the local ethics committee of the University Erlangen. Procedures were conducted in accordance with the Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schult, D., Hölsken, A., Buchfelder, M. et al. Expression pattern of neuronal intermediate filament α-internexin in anterior pituitary gland and related tumors. Pituitary 18, 465–473 (2015). https://doi.org/10.1007/s11102-014-0597-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-014-0597-2