Abstract
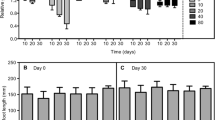
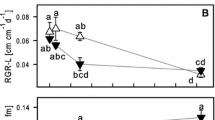
Arsenic is a critical contaminant that is released into the environment through geochemical processes and anthropic actions. Two independent hydroponic experiments were performed to evaluate the ecophysiological responses of water hyacinth [Eichhornia crassipes (Mart.) Solms] to As under various stress conditions. In experiment 1, water hyacinth was exposed to As5+ at concentrations of 0, 0.2, 2.0, and 20 mg L−1 for 0, 2, and 4 d; in experiment 2, water hyacinth was exposed at concentrations of 0, 0.025, 0.05, and 0.1 mg L−1 for 0, 10, and 20 d. In both experiments, As accumulation in plant tissue was proportional to its increase in the nutrient solution; As concentrations were higher in roots than in shoots. Detrimental effects of As on gas exchange were observed and were more pronounced in experiment 1. In experiment 1, at the beginning on the second day of exposure, significant decreases of maximum photochemical efficiency of PSII (Fv/Fm), variable chlorophyll fluorescence (Fv/F0), and photosynthetic pigment contents were observed in plants exposed to 2.0 and 20 mg(As5+) L−1. It indicated that damage to the photosynthetic apparatus had occurred. No changes in Fv/Fm, Fv/F0, and contents of photosynthetic pigments were observed in the plants grown in the presence of 0.2 mg(As5+) L−1 (in experiment 1) or after any of the treatments in experiment 2, indicating plant tolerance. Elevated nonphotochemical quenching was observed in experiment 2 after 20 d of exposure to As; it was as a part of protection mechanisms of the photosynthetic apparatus in these plants. The results obtained here indicate that the use of water hyacinth for As5+ removal from highly impacted environments is limited but that it is effective in remediating sites with a low contamination.
Similar content being viewed by others
Abbreviations
- Car:
-
carotenoids
- Chl:
-
chlorophyll
- C i :
-
intercellular CO2 concentration
- DMSO:
-
dimethylsulfoxide
- F0 :
-
minimal fluorescence yield of the dark-adapted state
- Fm :
-
maximal fluorescence yield of the dark-adapted state
- Fv :
-
variable fluorescence
- Fv/F0 :
-
variable chlorophyll fluorescence
- Fv/Fm :
-
maximum photochemical efficiency of PSII
- g s :
-
stomatal conductance
- NPQ:
-
nonphotochemical quenching
- P N :
-
net photosynthetic rate
- qP :
-
photochemical quenching
References
Azcue J.M., Nriagu J.O.: Impact of abandoned mine tailings on the arsenic concentrations in Moira Lake, Ontario. — J. Geochem. Explor. 52: 81–89, 1995.
Björkman O., Demmig B.: Photon yield of oxygen evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origin. — Planta 170: 489–504, 1987.
Bolhar-Nordenkampf H.R., Long S.P., Baker N.R.: Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: a review of current instrumentation. — Func. Ecol. 3: 497–514, 1989.
Borba R.P., Figueiredo B.R., Rawlins B., Matschullat J.: Geochemical distribution of arsenic in waters, sediments and weathered gold mineralized rocks from Iron Quadrangle, Brazil. — Environ. Geol. 44: 39–52, 2003.
Borba R.P., Figueiredo B.R., Cavalcanti J.A.: [Arsenic in groundwater in Ouro Preto and Mariana, Iron Quadrangle (MG).] — Geociências 57: 45–51, 2004. [In Portuguese]
Burns J., Fraser P.D., Bramley P.M.: Identification and quantification of carotenoids, tocopherols and chlorophyll in commonly consumed fruits and vegetables. — Phytochemistry 62: 939–947, 2003.
CONAMA nº 357. March 17. Published in DOU nº 53, march 18. Section 1, 58–63, Ministério do Meio Ambiente, 2005. [In Portuguese]
Demmig-Adams B., Adams W.W.: The role of xanthophyll cycle carotenoids in the protection of photosynthesis. — Trend. Plant Sci. 1: 21–26, 1996.
Farnese F.S., Oliveira J.A., Gusman G.S. et al.: Effects of adding nitroprusside on arsenic stressed response of Pistia stratiotes L. under hydroponic conditions. — Int. J. Phyto-remediat. 16: 123–137, 2014.
Garg N., Singla P.: Arsenic toxicity in crop plants: physiological effects and tolerance mechanisms. — Environ. Chem. Lett. 9: 303–321, 2011.
Genty B., Briantais J.M., Baker N.R.: The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. — Biochim. Biophys. Acta 990: 87–92, 1989.
Giri A.K., Patel R.K.: Phytoaccumulation potential and toxicity of arsenic ions by Eichhornia crassipes in hydroponic system. — J. Bioremed. Biodegrad. 3: 2–6, 2012.
Gonçalves J.A.C., Lena J.C., Paiva J.F. et al.: Arsenic in the groundwater of Ouro Preto (Brazil): its temporal behavior as influenced by the hydric regime and hydrogeology. — Environ. Geol. 53: 785–793, 2007.
Gusman G.S., Oliveira J.A., Farnese F.S. et al.: Arsenate and arsenite: the toxic effects on photosynthesis and growth of lettuce plants. — Acta Physiol. Plant. 35: 1201–1209, 2013.
He J., Lee S.K., Dodd I.C.: Limitations to photosynthesis of lettuce growth under tropical conditions: alleviation by rootzone cooling. — J. Exp. Bot. 52: 1323–1330, 2001.
Hoagland D.R., Arnon D.I.: The Water Culture Method for Growing Plants without Soil. Pp. 34. California Agriculture Station, Berkeley 1950.
Iriel A., Dundas G., Cirelli A.F. et al.: Effect of arsenic on reflectance spectra and chlorophyll fluorescence of aquatic plants. — Chemosphere 119: 697–703, 2015.
Jain C.K., Ali I.: Arsenic: occurrence, toxicity and speciation techniques. — Water Res. 34: 4304–4312, 2000.
Jana S.: Accumulation of Hg and Cr by three aquatic species and subsequent changes in several physiological and bio-chemical plant parameters. — Water Air Soil Poll. 38: 105–109, 1988.
Jiang Q.Q., Singh B.R.: Effect of different forms and sources of arsenic on crop yield and arsenic concentration. — Water Air Soil Poll. 74: 321–343, 1994.
Karimi N., Shayesteh L.S., Ghasmpour H. et al.: Effects of arsenic on growth, photosynthetic activity, and accumulation in two new hyperaccumulating populations of Isatis cappadocica Desv. — J. Plant Growth Regul. 32: 823–830, 2013.
Krause G.H., Weis E.: Chlorophyll fluorescence and photosynthesis: the basics. — Annu. Rev. Plant Physiol. Plant Mol. Biol. 42: 313–349, 1991.
Lage-Pinto F., Oliveira J.G., Cunha M.D. et al.: Chlorophyll a fluorescence and ultrastructural changes in chloroplast of water hyacinth as indicators of environmental stress. — Environ. Exp. Bot. 64: 307–313, 2008.
Lichtenthaler H.K., Kuhn G., Prenzel U. et al.: Chlorophyll-protein levels and degree of thylakoid stacking in radish chloroplasts from high-light, low-light and bentazon-treated plants. — Physiol. Plantarum 56: 183–188, 2006.
Li X.P., Björkman O., Shih C. et al.: A pigment-binding protein essential for regulation of photosynthetic light harvesting. — Nature 403: 391–395, 2000.
Li M., Zhang J., Ling T. et al.: Ecophysiological responses of Jussiaea rapens to cadmium exposure. — Aquat. Bot. 88: 347–352, 2008.
Ma L.Q., Komar K.M., Tu C. et al.: A fern that hyperaccumulates arsenic. — Nature 409: 579–579, 2001.
Maleva M.G., Nekrasova G.F., Borisova G.G. et al.: Effect of heavy metals on photosynthetic apparatus and antioxidant status of Elodea. — Russ. J. Plant Physl+ 59: 190–197, 2012.
Martínez-Sánchez M.J., Martínez-López S., García-Lorenzo M.L. et al.: Evaluation of arsenic in soils and plant uptake using various chemical extraction methods in soils affected by old mining activities. — Geoderma 160: 535–541, 2011.
Mathews S., Ma L.Q., Rathinasabapathi B. et al.: Arsenic transformation in the growth media and biomass of hyperaccumulator Pteris vittata L. — Bioresource Technol. 101: 8024–8030, 2010.
Meharg A.A., Hartley-Whitaker J.: Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. — New Phytol. 154: 29–43, 2002.
Oliveira J.G., Alves P.L.C.A., Magalhães A.C.N.: The effect of chilling on the photosynthetic activity in coffee (Coffea arabica L.) seedlings. The protective action of chloroplastid pigments. — Braz. J. Plant Physiol. 14: 95–104, 2002.
Paiva L.B., Oliveira J.G., Azevedo R.A. et al.: Ecophysiological responses of water hyacinth exposed to Cr3+ and Cr6+. — Environ. Exp. Bot. 65: 403–409, 2009.
Päivöke A.E.A., Simola L.K.: Arsenate toxicity to Pisum sativum: mineral nutrients, chlorophyll content and phytase activity. — Ecotox. Environ. Safe. 49: 111–121, 2001.
Pereira F.J., Castro E.M., Oliveira C. et al.: [Anatomical and physiological mechanisms of water hyacinth plants to arsenic contamination tolerance.] — Planta Daninha 29: 259–267, 2011. [In Portuguese]
Rabelo G.R., Vitória A.P., da Silva M.V.A. et al.: Structural and ecophysiological adaptations to forest gaps. — Trees-Struct. Funct. 27: 259–272, 2013.
Rezende P.S., Moura P.A.S., Durão W.A.J. et al.: Arsenic and mercury mobility in Brazilian sediments from the São Francisco River Basin. — J. Braz. Chem. Soc. 22: 910–918, 2011.
Robinson B., Duwing C., Bolan N. et al.: Uptake of arsenic by New Zealand watercress (Lepidium sativum). — Sci. Total Environ. 301: 67–73, 2003.
Rohácek K.: Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning and mutual relationships. — Photosynthetica 40: 13–29, 2002.
Salt D.E., Smith R.D., Raskin I.: Phytoremediation. — Annu. Rev. Plant Phys. 49: 643–668, 1998.
Schmöger M., Oven M., Grill E.: Detoxification of arsenic by phytochelatins in plants. — Plant Physiol. 122: 793–802, 2000.
Schützendübel A., Polle A.: Plant responses to abiotic stress: heavy metal-induced oxidative stress and protection by mycorrhization. — J. Exp. Bot. 53: 1351–1365, 2002.
Singh N.K., Pandey G.C., Rai U.N. et al.: Metal accumulation and ecophysiological effects of distillery effluent on Potamogeton pectinatus L. — Bull. Environ. Contam. Toxicol. 74: 857–863, 2005.
Singh N., Ma L.Q., Srivastava M. et al.: Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. — Plant Sci. 170: 274–282, 2006.
Singh N., Ma L.Q., Va J.C. et al.: Effects of arsenic on nitrate metabolism in arsenic hyperaccumulating and non-hyperaccumulating ferns. — Environ. Pollut. 157: 2300–2305, 2009.
Smart R.M., Barko J.W.: Laboratory culture of submersed freshwater macrophytes on natural sediments. — Aquat. Bot. 21: 251–263, 1985.
Smedley P.L., Kinniburgh D.G.: Sources and Behavior of Arsenic in Natural Water. United Nations Synthesis Report on Arsenic in Drinking Water. Pp. 61. WHO, Geneva 2001.
Soltan M.E., Rashed M.N.: Laboratory study on the survival of water hyacinth under several conditions of heavy metal concentrations. — Adv. Environ. Res. 7: 321–334, 2003.
Souza V.L., Silva D.C., Santana K.B. et al.: Effects of cadmium on the anatomy and photosynthesis of two aquatic macrophytes. — Acta Bot. Bras. 23: 342–354, 2009.
Srivastava S., Mishra S., Tripathi R.D. et al.: Phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (L.f.) Royle. — Environ. Sci. Technol. 41: 2930–2936, 2007.
Stoeva N., Bineva T.: Oxidative changes and photosynthesis in oat plants grown in As-contaminated soil. — Bulgarian J. Agric. Sci. 29: 87–95, 2003.
Stoeva N., Berova M., Zlatev Z.: Physiological response of maize to arsenic contamination. — Biol. Plantarum 47: 449–452, 2003.
Stoeva N., Berova M., Zlatev Z.: Effect of arsenic on some physiological parameters in bean plants. — Biol. Plantarum 49: 293–296, 2005.
van Kooten O., Snel J.F.H.: The use of chlorophyll fluorescence nomenclature in plant stress physiology. — Photosynth. Res. 25: 147–150, 1990.
Verkley J.A.C., Schat H.: Mechanisms of metal tolerance in higher plants. — In: Shaw A.J. (ed.): Heavy Metal Tolerance in Plants: Evolutionary Aspects. Pp. 179–193. CRC Press, New York 1992.
Vieira T.O., Degli-Espositi M.S.O., Souza G.M. et al.: Photoacclimation capacity in seedling and sapling of Siparuna guianensis (Siparunaeae): Response to irradiance gradient in tropical forest. — Photosynthetica 53: 11–22, 2015.
Vitória A.P., Lage-Pinto F., Campaneli L.B. et al.: Ecophysiological adaptation and metal accumulation in water hyacinth from two tropical rivers. — Braz. J. Plant. Physiol. 22: 49–59, 2010.
Vitória A.P., Lage-Pinto F., Campaneli L.B. et al.: Structural and ecophysiological alterations of the water hyacinth [Eichhornia crassipes (Mart.) Solms] due to anthropogenic stress in Brazilian rivers. — Braz. Arch. Biol. Technol. 54: 1059–1068, 2011.
Vitória, A.P., Santos, J.L.S., Salomão, M.S.M. B. et al.: Influence of ecologic type, seasonality, and origin of macrophyte in metal accumulation, anatomy and ecophysiology of Eichhornia crassipes and Eichhornia azurea. — Aquat. Bot. 125: 9–16, 2015.
Xue P.Y., Yan C.Z.: Arsenic accumulation and translocation in the submerged macrophyte Hydrilla verticillata (L.f.) Royle. — Chemosphere 85: 1176–1181, 2011.
Wellburn A.R.: The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. — J. Plant Physiol. 144: 307–313, 1994.
Zhao F.J., Ma J.F., Meharq A.A. et al.: Arsenic uptake and metabolism in plants. — New Phytol. 181: 777–794, 2009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: The authors wish to thank the Coordination for the Improvement of Higher Education Personnel (CAPES), Research Foundation for the State of the Rio de Janeiro (FAPERJ), and the State University of the North Fluminense Darcy Ribeiro for financial support.
Rights and permissions
About this article
Cite this article
Meneguelli-Souza, A.C., Vitória, A.P., Vieira, T.O. et al. Ecophysiological responses of Eichhornia crassipes (Mart.) Solms to As5+ under different stress conditions. Photosynthetica 54, 243–250 (2016). https://doi.org/10.1007/s11099-015-0174-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-015-0174-6