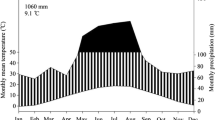
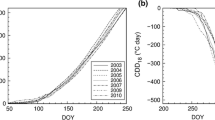
Abstract
Tropical canopy tree species can be classified into two types by their heterobaric and homobaric leaves. We studied the relation between both leaf types and their water use, together with the morphological characteristics of leaves and xylem, in 23 canopy species in a tropical rain forest. The maximum rates of photosynthesis and transpiration were significantly higher in heterobaric leaf species, which also underwent larger diurnal variations of leaf water potential compared to homobaric leaf species. The vessel diameter was significantly larger and the stomatal pore index (SPI) was significantly higher in heterobaric than that in homobaric leaf species. There was a significant positive correlation between the vessel diameter, SPI, and maximum transpiration rates in all the studied species of both leaf types. However, there was no significant difference in other properties, such as leaf water-use efficiency, leaf mass per area, leaf nitrogen content, and leaf δ13C between heterobaric and homobaric leaf species. Our results indicate that leaf and xylem morphological differences between heterobaric and homobaric leaf species are closely related to leaf water-use characteristics, even in the same habitat: heterobaric leaf species achieved a high carbon gain with large water use under strong light conditions, whereas homobaric leaf species can maintain a high leaf water potential even at midday as a result of low water use in the canopy environment.
Similar content being viewed by others
Abbreviations
- BSE:
-
bundle-sheath extension
- DBH:
-
stem diameter at breast height
- D v :
-
vessel diameter
- E max :
-
maximum transpiration rate
- GCL:
-
guard cell length
- g s :
-
stomatal conductance at P max
- LMA:
-
leaf mass per area
- Narea :
-
nitrogen per unit leaf area
- Nmass :
-
mass-based leaf nitrogen concentration
- PIC:
-
phylogenetically independent contrast
- P max :
-
maximum photosynthetic rate
- PNUE:
-
the ratio of CO2 assimilation rate to leaf organic nitrogen content
- RGR:
-
relative growth rate
- SD:
-
stomatal density
- SPI:
-
stomatal pore index
- V d :
-
vessel density
- VPD:
-
vapor pressure deficit
- W d :
-
wood density
- WUE:
-
intrinsic water-use efficiency
- δ13C:
-
stable carbon isotope ratio
- ΔΨL :
-
diurnal variation in the leaf water potential
- Ψmid :
-
leaf water potential at midday
- Ψpd :
-
leaf water potential at predawn
References
Anfodillo T., Carraro V., Carrer M. et al.: Convergent tapering of xylem conduits in different woody species. — New Phytol. 169: 279–290, 2006.
Ashton P.S.: Lambir’s Forest: The world’s most dive known tree assemblage? — In: Roubik D.W., Sakai S., Hamid Karim A.A. (ed.): Pollination Ecology and the Rain Forest. Pp. 191–216. Springer, New York 2005.
Bebber D.P.: Dipterocarp susceptibility to drought: A role for wood structure? — J. Trop. For. Sci. 14: 425–427, 2002.
Beyschlag W., Eckstein J.: Towards a causal analysis of stomatal patchiness: The role of stomatal size variability and hydrological heterogeneity. — Acta Oecol. 22: 161–173, 2001.
Boyce C.K., Brodribb T.J., Field T.S. Zwieniecki M.A.: Angiosperm leaf vein evolution was physiologically and environmentally transformative. — Proc. R. Soc. Ser. B-Bio. 276: 1771–1776, 2009.
Brodribb T.J., Bowman D.J.M.S., Nichols S. et al.: Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. — New Phytol. 188: 533–542, 2010a.
Brodribb T.J., Field T.S.: Stem hydraulic supply is linked to leaf photosynthetic capacity: Evidence from New Caledonian and Tasmanian rainforests. — Plant Cell Environ. 23: 1381–1388, 2000.
Brodribb T.J., Field T.S., Sack L.: Viewing leaf structure and evolution from a hydraulic perspective. — Funct. Plant Biol. 37: 488–498, 2010b.
Brodribb T.J., Jordan G.J.: Water supply and demand remain balanced during leaf acclimation of Nothofagus cunninghamii trees. — New Phytol. 192: 437–448, 2011.
Brodribb T.J., Jordan G.J., Carpenter R.J.: Unified changes in cell size permit coordinated leaf evolution. — New Phytol. 199: 559–570, 2013.
Buck A.L.: New equations for computing vapour pressure and enhancement factor. — J. Appl. Meteorol. 20: 1527–1532, 1981.
Buckley T.N., Miller J.M., Farquhar G.D.: The mathematics of linked optimisation for water and nitrogen use in a canopy. — Silva Fenn. 36: 639–669, 2002.
Buckley T.N., Sack L., Gilbert M.E.: The role of bundle sheath extensions and life form in stomatal responses to leaf water status. — Plant Physiol. 156: 962–973, 2011.
Cai J., Tyree M.T.: The impact of vessel size on vulnerability curves: Data and models for within-species variability in saplings of aspen, Populus tremuloides Michx. — Plant Cell Environ. 33: 1059–1069, 2010.
Christensen J.H., Hewitson B., Busuioc A. et al.: Regional climate projections In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. — In: Solomon S., Qin D., Manning M. et al. (ed.): Climate Change 2007. Pp. 847–940. Cambridge University Press, Cambridge and New York 2007.
Christman M.A., Sperry J.S., Smith D.D.: Rare pits, large vessels and extreme vulnerability to cavitation in a ring-porous tree species. — New Phytol. 193: 713–720, 2012.
Corlett R.T., Lafrankie J.V.: Potential impacts of climate change on tropical Asian forests through an influence on phenology. — Clim. Chang. 39: 439–453, 1998.
Crocker, WM.: Aeration systems of leaves. — Bot. Gaz. 67: 517–518, 1919.
Engelbrecht B.M.J., Comita L.S., Condit R. et al.: Drought sensitivity shapes species distribution patterns in tropical forests. — Nature 447: 80–82, 2007.
Hacke U.G., Sperry J.S., Wheeler J.K., Castro L.: Scaling of angiosperm xylem structure with safety and efficiency. — Tree Physiol. 26: 689–701, 2006.
Hiromi T., Ichie T., Kenzo T. et al.: Interspecific variation in leaf water use associated with drought tolerance in four emergent dipterocarp species of a tropical rain forest in Borneo. — J. Forest Res.-Jpn. 17: 369–377, 2012.
Hiromi T., Ninomiya I., Koike T. et al.: [Regulation of transpiration by patchy stomatal opening in canopy tree species of Dipterocarpaceae in tropical rainforest, Sarawak, Malaysia.] — Jap. J. Ecol. 49: 83–91, 1999. [In Japanese with English abstract]
Ishida A., Nakano T., Yazaki K. et al.: Coordination between leaf and stem traits related to leaf carbon gain and hydraulics across 32 drought-tolerant angiosperms. — Oecologia 156: 193–202, 2008.
Ishida A., Toma, T., Matsumoto Y. et al.: Diurnal changes in leaf gas exchange characteristics in the uppermost canopy of a rain forest tree, Dryobalanops aromatica Gaertn. f. — Tree Physiol. 16: 779–785, 1996.
Jones H.G.: Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. — Plant Cell Environ. 22: 1043–1055, 1999.
Kamakura M., Kosugi Y., Takanashi S. et al.: Patchy stomatal behavior during midday depression of leaf CO2 exchange in tropical trees. — Tree Physiol. 31: 160–168, 2011.
Karabourniotis G., Bornman J.F., Nikolopoulos D.: A possible optical role of the bundle sheath extensions of the heterobaric leaves of Vitis vinifera and Quercus coccifera. — Plant Cell Environ. 23: 423–430, 2000.
Kenzo T., Ichie T., Ninomiya I., Koike T.: Photosynthetic activity in seed wings of Dipterocarpaceae in a masting year: Does wing photosynthesis contribute to reproduction? — Photosynthetica 41: 551–557, 2003.
Kenzo T., Ichie T., Watanabe Y., Hiromi T.: Ecological distribution of homobaric and heterobaric leaves in tree species of Malaysian lowland tropical rainforest. — Am. J. Bot. 94: 764–775, 2007.
Kenzo T., Ichie T., Watanabe Y. et al.: Changes in photosynthesis and leaf characteristics with tree height in five dipterocarp species in a tropical rain forest. — Tree Physiol. 26: 865–873, 2006.
Kenzo T., Ichie T., Yoneda R. et al.: Interspecific variation of photosynthesis and leaf characteristics in canopy trees of five species of Dipterocarpaceae in a tropical rain forest. — Tree Physiol. 24: 1187–1192, 2004.
Kimmins J.P.: Forest Ecology. A Foundation for Sustainable Management. — Prentice Hall, Upper Saddle River, New Jersey 1997.
Koike T., Miyashita N., Toda H.: Effect of leaf structural characteristics in successional deciduous broadleaved tree seedlings and their silvicultural meaning. — For. Resour. Environ. 35: 9–25, 1997.
Koike T., Watanabe T., Toda H., Haibara K.: Morphological diversity of stomata of representative broadleaved trees in a temperate region: detection with the Sump method. — For. Resour. Environ. 36: 55–63, 1998.
Kumagai T., Katul G.G., Saitoh T.M. et al.: Water cycling in a Bornean tropical rain forest under current and projected precipitation scenarios. — Water Resour. Res. 40: W01104, doi: 10.1029/2003WR002226, 2004.
Kumagai T., Kume T.: Influences of diurnal rainfall cycle on CO2 exchange over Bornean tropical rainforests. — Ecol. Model. 246: 91–98, 2012.
Kume T, Komatsu H, Kuraji K, Suzuki M.: Less than 20-min time lags between transpiration and stem sap flow in emergent trees in a Bornean tropical rainforest. — Agr. Forest Meteorol. 148: 1181–1189, 2008.
Kume T., Tanaka N., Kuraji K. et al.: Ten-year evapotranspiration estimates in a Bornean tropical rainforest. — Agr. Forest Meteorol. 151: 1183–1192, 2011.
Lawson T., Morison J.: Visualising patterns of CO2 diffusion in leaves. — New Phytol. 169: 641–643, 2006.
Liakoura V., Fotelli M.N., Rennenberg H., Karabourniotis G.: Should structure -function relations be considered separately for Homobaric vs. Heterobaric leaves? — Am. J. Bot. 96: 612–619, 2009.
Lintunen A., Kalliokoski T.: The effect of tree architecture on conduit diameter and frequency from small distal roots to branch tips in Betula pendula, Picea abies and Pinus sylvestris. — Tree Physiol. 30: 1433–1447, 2010.
Lynch D.J., McInerney F.A., Kouwenberg L.L.R. et al.: Plasticity in bundle sheath extensions of heterobaric leaves. — Am. J. Bot. 99: 1197–1206, 2012.
Manzoni S., Vico G., Katul G. et al.: Hydraulic limits on maximum plant transpiration and the emergence of the safetyefficiency trade-off. — New Phytol. 198: 169–178, 2013.
Martínez-Vilalta J., Prat E., Oliveras I. et al.: Xylem hydraulic properties of roots and stems of nine Mediterranean woody species. — Oecologia 133: 19–29, 2002.
McCulloh K., Sperry J.S., Lachenbruch B. et al.: Moving water well: Comparing hydraulic efficiency in twigs and trunks of coniferous, ring-porous, and diffuseporous saplings from temperate and tropical forests. — New Phytol. 186: 439–450, 2010.
McKown A.D., Cochard H., Sack L.: Decoding leaf hydraulics with a spatially explicit model: Principles of venation architecture and implications for its evolution. — Am. Nat. 175: 447–460, 2010.
Morison J.I.L., Lawson T., Cornic G.: Lateral CO2 diffusion inside dicotyledonous leaves can be substantial: Quantification in different light intensities. — Plant Physiol. 145: 680–690, 2007.
Mott K.A., Peak D.: Stomatal patchiness and task-performing networks. — Ann. Bot. 99: 219–226, 2007.
Nakagawa M., Momose K., Kishimoto-Yamada K. et al.: Tree community structure, dynamics, and diversity partitioning in a Bornean tropical forested landscape. — Biodivers. Conserv. 22: 127–140, 2013.
Nakagawa M., Tanaka K., Nakashizuka T. et al.: Impact of severe drought associated with the 1997–1998 El Nino in a tropical forest in Sarawak. — J. Trop. Ecol. 16: 355–367, 2000.
Nardini A., Gortan E., Salleo S.: Hydraulic efficiency of the leaf venation system in sun- and shade-adapted species. — Funct. Plant Biol. 32: 953–961, 2005.
Nikolopoulos D., Liakopoulos G., Drossopoulos I., Karabourniotis, G.: The relationship between anatomy and photosynthetic performance of heterobaric leaves. — Plant Physiol. 129: 235–243, 2002.
Nobel P.S.: Physicochemical and Environmental Plant Physiology, 2nd Ed. — Academic Press, San Diego 1999.
Osunkoya O.O., Sheng T.K., Mahmud N.A., Damit N.: Variation in wood density, wood water content, stem growth and mortality among twenty-seven tree species in a tropical rainforest on Borneo Island. — Austral Ecol. 32: 191–201, 2007.
Overpeck J.T., Cole J.E.: Climate change: Lessons from a distant monsoon. — Nature 445: 270–271, 2007.
Paradis E., Claude J., Strimmer K.: APE: analyses of phylogenetics and evolution in R language. — Bioinformatics 20: 289–290, 2004.
Pieruschka R., Chavarría-Krauser A., Cloos K. et al.: Photosynthesis can be enhanced by lateral CO2 diffusion inside leaves over distances of several millimeters. — New Phytol. 178: 335–347, 2008.
Pieruschka R., Chavarría-Krauser A., Schurr U., Jahnke S.: Photosynthesis in lightfleck areas of homobaric and heterobaric leaves. — J. Exp. Bot. 61: 1031–1039, 2010.
Pieruschka R., Schurr U., Jensen M. et al.: Lateral diffusion of CO2 from shaded to illuminated leaf parts affects photosynthesis inside homobaric leaves. — New Phytol. 169: 779–788, 2006.
Pons T.L., Welschen R.A.M.: Overestimation of respiration rates in commercially available clamp-on leaf chambers. Complications with measurement of net photosynthesis. — Plant Cell Environ. 25: 1367–1372, 2002.
Roth I.: Stratification of Tropical Forests as Seen in Leaf Structure. — Springer Netherlands, The Hague 1984.
Sack L., Cowan P.D., Jaikumar N., Holbrook N.M.: The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. — Plant Cell Environ. 26: 1343–1356, 2003.
Sack L., Frole K.: Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. — Ecology 87: 483–491, 2006.
Sack L., Tyree M.T., Holbrook N.M.: Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. — New Phytol. 167: 403–413, 2005.
Sakai S., Nakashizuka T., Ichie T. et al.: Lambir Hills canopy crane, Malaysia. — In: Mitchell A.W., Secoy K., Jackson T. (ed.): The Global Canopy Handbook. Pp. 77–79. Global Canopy Programme, Oxford 2002.
Santiago L.S., Goldstein G., Meinzer F.C. et al.: Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. — Oecologia 140: 543–550, 2004.
Scoffoni C., Pou A., Aasamaa K., Sack L.: The rapid light response of leaf hydraulic conductance: New evidence from two experimental methods. — Plant Cell Environ. 31: 1803–1812, 2008.
Schuldt B., Leuschner C., Brock N., Horna V.: Changes in wood density, wood anatomy and hydraulic properties of the xylem along the root-to-shoot flow path in tropical rainforest trees. — Tree Physiol. 33: 161–174, 2013.
Takanashi S., Kosugi Y., Matsuo N. et al.: Patchy stomatal behavior in broad-leaved trees grown in different habitats. — Tree Physiol. 26: 1565–1578, 2006.
Tanaka Y., Sugano S.S., Shimada T., Hara-Nishimura I.: Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. — New Phytol. 198: 757–764, 2013.
Tanaka-Oda A., Kenzo T., Koretsune S. et al.: Ontogenetic changes in water-use efficiency (δ13C) and leaf traits differ among tree species growing in a semiarid region of the Loess Plateau, China. — Forest Ecol. Manag. 259: 953–957, 2010.
Terashima I.: Anatomy of non-uniform leaf photosynthesis. — Photosynth. Res. 31: 195–212, 1992.
Tsumura Y., Kado T., Yoshida K. et al.: Molecular database for classifying Shorea species (Dipterocarpaceae) and techniques for checking the legitimacy of timber and wood products. — J. Plant Res. 124: 35–48, 2011.
Tyree M.T., Dixon M.A.: Water stress induced cavitation and embolism in some woody plants. — Physiol. Plantarum 66: 397–405, 1986.
Walls R.L.: Angiosperm leaf vein patterns are linked to leaf functions in a global-scale data set. — Am. J. Bot. 98: 244–253, 2011.
Webb C.O., Ackerly D.D., Kembel S.W.: Phylocom: software for the analysis of phylogenetic community structure and trait evolution. — Bioinformatics 24: 2098–2100, 2008.
West J.D., Peak D., Peterson J.Q., Mott K.A.: Dynamics of stomatal patches for a single surface of Xanthium strumarium L. leaves observed with fluorescence and thermal images. — Plant Cell Environ. 28: 633–641, 2005.
Wheeler J.K., Sperry J.S., Hacke U.G., Hoang N.: Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: A basis for a safety versus efficiency trade-off in xylem transport. — Plant Cell Environ. 28: 800–812, 2005.
Whitmore, T.C.: An Introduction to Tropical Rain Forest, 2nd Ed. — Oxford University Press, Oxford 1998.
Wright S.J.: The future of tropical forests. — Ann. N.Y. Acad. Sci. 1195: 1–27, 2010.
Wylie R.B.: Principles of foliar organization shown by sun-shade leaves from ten species of deciduous dicotyledonous trees. — Am. J. Bot. 38: 355–361, 1951.
Wylie R.B.: The bundle sheath extension in leaves of dicotyledons. — Am. J. Bot. 39: 645–651, 1952.
Xu Z., Zhou G.: Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. — J. Exp. Bot. 59: 3317–3325, 2008.
Yáñez-Espinosa L., Terrazas T., López-Mata L., Valdez-Hernández J.I.: Leaf trait variation in three species through canopy strata in a semi-evergreen neotropical forest. — Can. J. Bot. 81: 398–404, 2003.
Yoda K.: Three-dimensional distribution of light intensity in a tropical rain forest of West Malaysia. — Jpn. J. Ecol. 24: 247–254, 1974.
Zach A., Schuldt B., Brix S. et al.: Vessel diameter and xylem hydraulic conductivity increase with tree height in tropical rainforest trees in Sulawesi, Indonesia. — Flora 205: 506–512, 2010.
Zhang J.L., Cao K.F.: Stem hydraulics mediates leaf water status, carbon gain, nutrient use efficiencies and plant growth rates across dipterocarp species. — Funct. Ecol. 23: 658–667, 2009.
Zwieniecki M.A., Brodribb T.J., Holbrook N.M.: Hydraulic design of leaves: Insights from rehydration kinetics. — Plant Cell Environ. 30: 910–921, 2007.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: We are grateful to the Forest Department, Sarawak, and Prof. N. Yamamura for their kind support of this study. We thank Prof. S. Fujiwara for his help with microscopic analysis, Dr. M. Nakagawa for providing tree census data, and Dr. Kume for providing meteorological data. We also thank two anonymous reviewers for constructive critique. This research was partly supported by the Grant-in-Aid for Scientific Research from JSPS (No. 23255002, 244050321), the Research Institute for Humanity and Nature (project No. D-04), and the Environment Research and Technology Development Fund (RF-1010, S-9) of the Ministry of the Environment, Japan.
Rights and permissions
About this article
Cite this article
Inoue, Y., Kenzo, T., Tanaka-Oda, A. et al. Leaf water use in heterobaric and homobaric leafed canopy tree species in a Malaysian tropical rain forest. Photosynthetica 53, 177–186 (2015). https://doi.org/10.1007/s11099-015-0105-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-015-0105-6